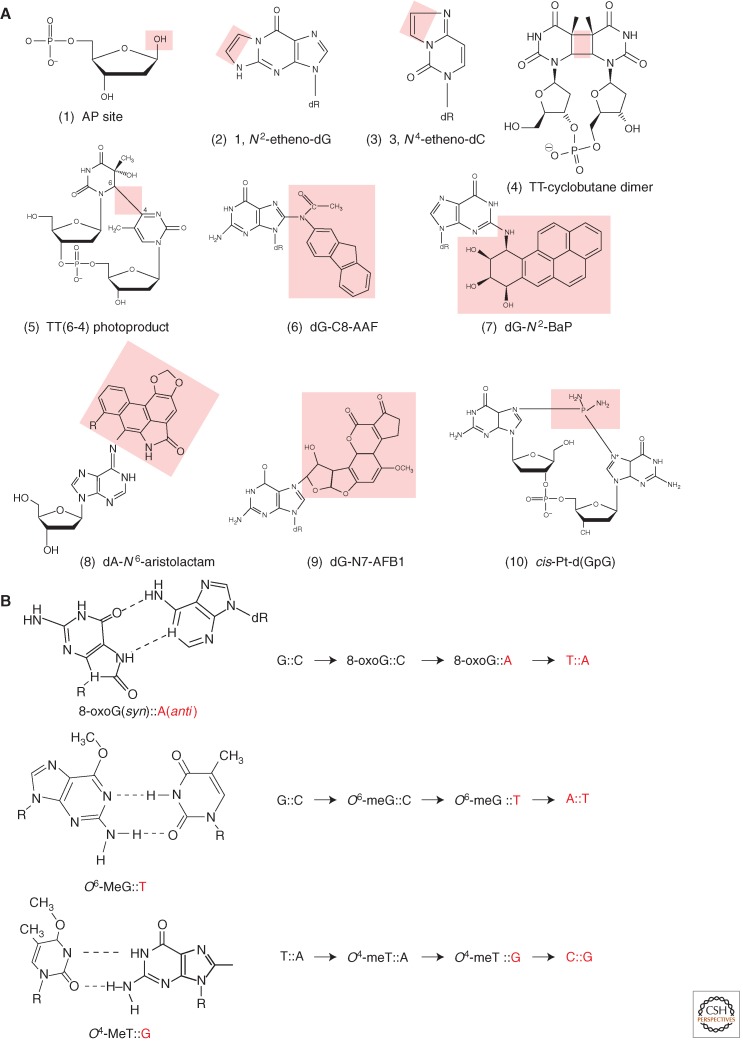
Figure 1.
Diversity of DNA lesions and properties of common miscoding lesions. (A) A glance at the huge diversity of chemical lesions in DNA: Lesions are highlighted by the pink area. (1) Abasic site, a common lesion that can be formed in a variety of ways: spontaneous or alkylation-induced depurination, repair intermediates. (2, 3) G and C etheno-type adducts are formed by various chemicals such as vinyl chloride, lipid peroxidation: A new cycle is formed by double adduction at an exocyclic and an intracyclic nitrogen atom. (4, 5) TT-cyclobutane dimer (CPD) and T(6–4)T photoproduct formed by UV light. (6) dG-C8-AAF is a major adduct formed by an aromatic amine N-2-acetylaminofluorene (AAF), a strong liver carcinogen. (7) B(a)P-N2-dG, the major guanine adduct formed by benzo(a)pyrene, a common polycyclic hydrocarbon found in cigarette smoke and other combustion residues. (8) dA-N6-Aristolactam, an adduct formed by a metabolite of Aristolochia clematitis, a plant that often grows in cultivated fields where its seeds comingle with wheat grain during harvest (Grollman et al. 2007). (9) dG-N7-AFB1 is the major guanine adduct of a potent hepatocarcinogen. Aflatoxin B1, a metabolite produced by a mold that grows on peanuts. (10) cis-Pt-d(GpG) is an intrastrand cross-link produced by the drug cisplatin that is used in human cancer chemotherapy. (B) Examples of direct miscoding lesions. These lesions do not block replicative DNA polymerases. Instead, replicative DNA polymerases efficiently insert a nucleotide opposite these lesions forming a noncanonical base pair that leads to a base substitution at the next replication cycle, as discussed in the text. The nucleotides introduced during replication are shown in red.