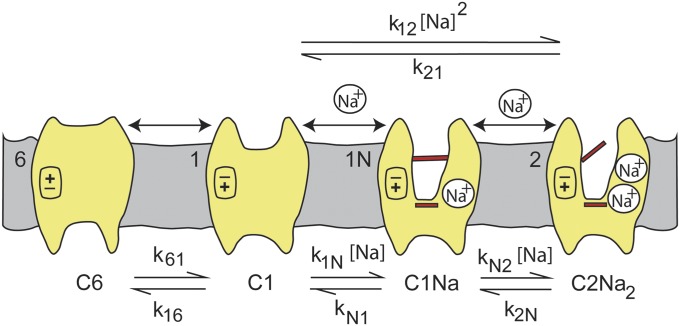
Fig. 1.
Cartoon representing three- and four-state models for Na+ binding to SGLT1. For clarity of presentation, we assume the number (n) of Na+ ions binding to the empty transporter is 2. The ligand-free protein carries a net negative charge and transient capacitive SGLT1 currents are associated with reorientation of the empty transporter between external and internal membrane surfaces (C1 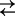 C6) and Na+ binding/dissociation (C1 + 2Na+
C6) and Na+ binding/dissociation (C1 + 2Na+
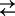 C2Na2 for the three-state and C1+Na+
C2Na2 for the three-state and C1+Na+
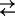 C1Na + Na+
C1Na + Na+
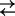 C2Na2 for the four-state model). Kd is the lumped sodium dissociation constant for the three-state model, and KdA and KdB are the sodium dissociation constants for the first and second binding sites for the four-state model (Theory section). Hyperpolarizing voltages drive the transporter to the outward conformation (C6 → C1), as well as promoting the transition C1 → C2Na2.
C2Na2 for the four-state model). Kd is the lumped sodium dissociation constant for the three-state model, and KdA and KdB are the sodium dissociation constants for the first and second binding sites for the four-state model (Theory section). Hyperpolarizing voltages drive the transporter to the outward conformation (C6 → C1), as well as promoting the transition C1 → C2Na2.