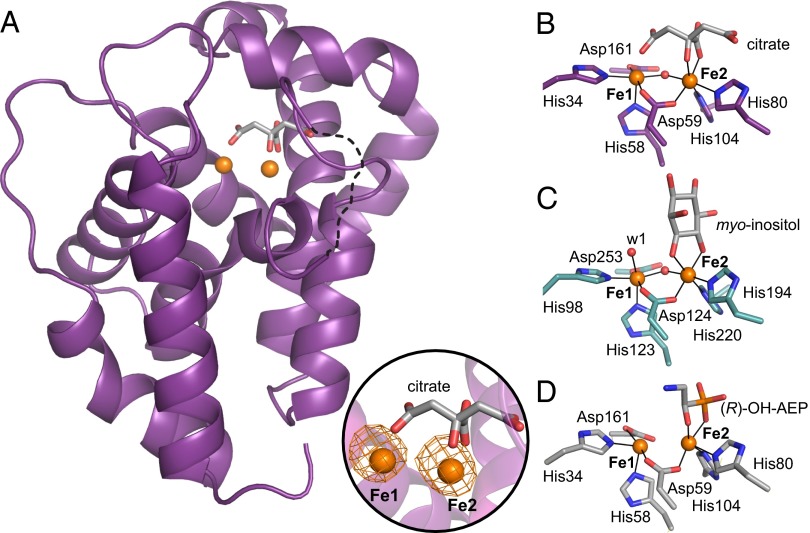
Fig. 3.
Three-dimensional structures of PhnZ shown in comparison with MIOX. (A) Ribbon diagram illustrating the α-helical fold of PhnZ, the location of the diiron site, and coordination by a citrate molecule. A loop region (residues 66–72) that could not be modeled is represented as a dotted line. An Fe anomalous difference Fourier map (orange mesh, contoured at 7.0 σ) is shown in Inset. Also shown are views of the active site in PhnZ with the citrate ligand (B), MIOX with the substrate myo-inositol (C), and PhnZ with the substrate (R)-OH-AEP (D). Amino acid side chains in the first coordination sphere and substrate molecules are represented in stick format, FeIII ions are shown as orange spheres, and nonprotein oxygen-based ligands are shown as red spheres. Because of the modest resolution (3.0 Å) of the (R)-OH-AEP–bound PhnZ structure, the oxo/hydoxo bridge cannot be resolved and, therefore, was not modeled.