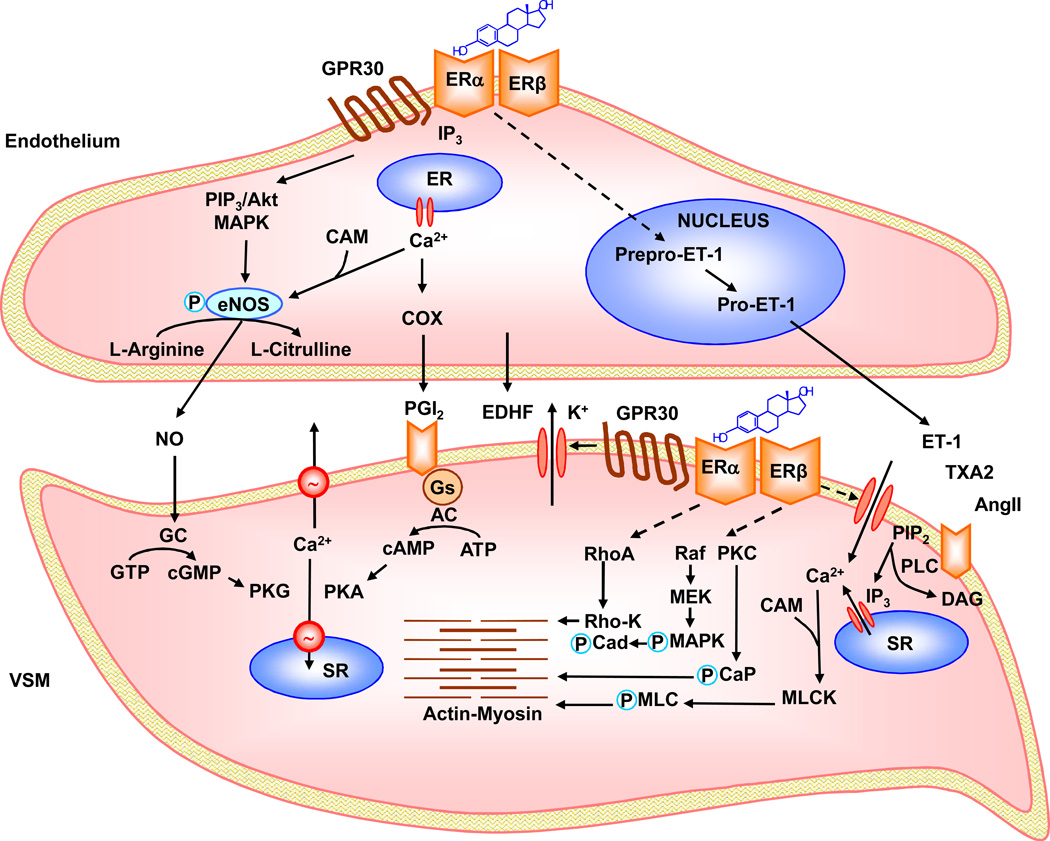
Fig. 2.
Estrogen-induced ER-mediated pathways of vascular relaxation. Estrogens such 17β-estradiol (E2) binds to endothelial ERα, ERβ or GPR30 and activates phospholipase C (PLC), leading to the generation of inositol 1,4,5- triphosphate (IP3) and diacylglycerol (DAG). IP3 causes the release of Ca2+ from the endoplasmic reticulum (ER). Ca2+ forms a complex with calmodulin (CAM), which causes initial activation of eNOS. E2 also activates phosphatidylinositol 3-kinase (PI3K), which transforms phosphatidylinositol-4,5-bisphosphate (PIP2) into phosphatidylinositol 3,4,5-trisphosphate (PIP3), and activates Akt. ER-mediated activation of Akt or MAPK causes phosphorylation and full activation of eNOS. Fully activated eNOS transforms L-arginine to L-citrulline and produces NO, which diffuses through ECs and activates guanylate cyclase in VSM leading to increased cGMP and stimulation of cGMP-dependent protein kinase (PKG). PKG decreases [Ca2+]i by stimulating Ca2+ extrusion pumps in the plasma membrane and Ca2+ uptake pumps in SR and/or decreases the sensitivity of the contractile myofilaments to [Ca2+]i and thereby promotes VSM relaxation. E2 also activates COX to produce prostacyclin (PGI2) which activates cAMP-dependent pathway, protein kinase A (PKA), and promotes relaxation pathways similar to cGMP/PKG. E2 also induces the release of EDHF which activates K+ channels and causes hyperpolarization and relaxation of VSM. E2 could also decrease endothelial ET-1 expression and production via genomic pathway. In VSM, agonists such as ET-1, TXA2 and AngII activate specific VSM receptors, stimulate PLC, and increase the production of IP3 and DAG. IP3 stimulates Ca2+ release from the sarcoplasmic reticulum. Agonists also stimulate Ca2+ entry through Ca2+ channels. Ca2+ binds CAM, activates myosin light chain kinase (MLCK), causes MLC phosphorylation, and initiates actin-myocin interaction and VSM contraction. DAG activates PKC, which in turn phosphorylates calponin (CaP) and/or activate a protein kinase cascade involving Raf, MAPK kinase (MEK) and MAPK, leading to phosphorylation of caldesmon (CaD) and increased myofilament force sensitivity to Ca2+. E2 binds to VSM ERs, leading to inhibition of agonist-activated mechanisms of VSM contraction. E2 activates K+ channels, leading to membrane hyperpolarization and inhibition of Ca2+ entry through Ca2+ channels. E2 may also inhibit PKC, MAPK or the RhoA/Rho-kinase (Rho-K) [Ca2+]i sensitization pathway. Dashed lines indicate inhibition.