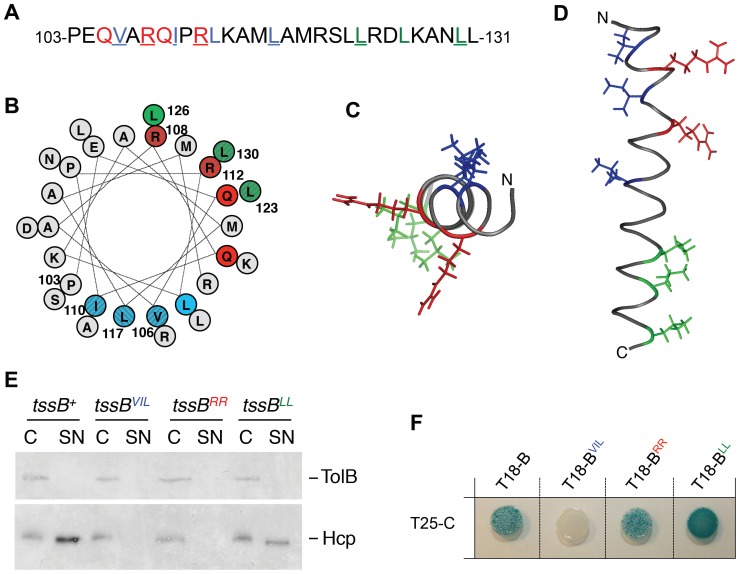
Figure 3. The hydrophobic motif of the TssB1 α-helix is required for T6SS function and interaction with TssC1.
Amino-acid sequence (A) and helical wheel projection (B) of the TssB1 α-helix (from residue Pro-103 to residue Leu-131). The different motifs described in this study are highlighted in different colours: blue, N-terminal hydrophobic; red, polar/charged; green, leucine-rich. The residues of these motifs mutagenized in this study are underlined (A) or striped (B). Top-view (C) and side-view (D) projections of the TssB1 α-helix. The targeted residues are colored as in panel (A). Top- and side-views have been modelled using PyMOL v0.99. (E) Hcp release assay. HcpFLAG release was assessed by separating whole cells (C) and culture supernatant (SN) fractions from 2×109 ΔtssB1 cells producing TssB1 (tssB+) or TssB1 bearing substitutions within the hydrophobic (tssBVIL), the polar/charged (tssBRR) or the leucin-rich (tssBLL) motif. Proteins were separated by 12.5%-acrylamide SDS-PAGE and Hcp and TolB were immunodetected using anti-FLAG monoclonal (lower panel) and anti-TolB polyclonal (upper panel) antibodies. (F) Bacterial two-hybrid assay. BTH101 reporter cells producing the T25 domain of the Bordetella adenylate cyclase fused to TssC1 (T25-C) and the T18 domain fused to TssB1 (T18-B) or TssB1 variants bearing substitutions within the hydrophobic (T18-BVIL), the polar/charged (T18-BRR) or the leucin-rich (T18-BLL) motif were spotted on X-Gal indicator LB agar plates.