Abstract
Purpose
The purpose of this study was to test the feasibility and preliminary effects of a culturally grounded, community-based diabetes prevention program among obese Latino adolescents.
Methods
Fifteen obese Latino adolescents (body mass index [BMI] percentile = 96.3 ± 1.1, age = 15.0 ± 0.9 years) completed a 12-week intervention that included weekly lifestyle education classes delivered by bilingual/bicultural promotoras and three, 60-minute physical activity sessions per week. Participants were assessed for anthropometrics (height, weight, BMI, and waist circumference), cardiorespiratory fitness, physical activity/inactivity, nutrition behaviors, and insulin sensitivity and glucose tolerance by a 2-hour oral glucose tolerance test.
Results
The intervention resulted in significant decreases in BMI z score, BMI percentile, and waist circumference; increases in cardiorespiratory fitness; and decreases in physical inactivity and dietary fat consumption. In addition to these changes, the intervention led to significant improvements in insulin sensitivity and reductions in 2-hour glucose levels.
Conclusions
These results support the feasibility and efficacy of a community-based diabetes prevention program for high-risk Latino youth. Translational approaches that are both culturally grounded and biologically meaningful represent a novel and innovative strategy for closing the obesity-related health disparities gap.
Obesity-related health disparities are a major public health challenge facing today’s youth. In particular, Latino youth are 1.5 to 1.7 times more likely to be obese relative to non-Hispanic whites, thus contributing to a disproportionate burden of risk for developing cardiovascular disease (CVD) and type 2 diabetes (T2D). Latino adolescents are significantly more insulin resistant than whites1 and exhibit the highest rates of metabolic syndrome among US adolescents.2 A recent cohort study suggests that up to 30% of overweight Latino youth have impaired glucose tolerance3 and approximately 30% exhibit the metabolic syndrome.4 Collectively, these findings support recent estimates by the CDC that up to 50% of Latino youth born in the year 2000 will develop T2D in their lifetime.5
Several large-scale randomized clinical trials have established that lifestyle modification can prevent or delay the onset of T2D in high-risk adults who have impairedglucosetolerance(pre-diabetes).6 Comparatively, the literature that describes diabetes prevention programs for youth is extremely limited.7 Most lifestyle interventions for children and adolescents target obesity rather than more proximal indicators of diabetes risks, such as insulin sensitivity or glucose tolerance.8 Moreover, very few interventions are culturally grounded to meet the specific needs of minority youth, for whom the incidence of T2D exceeds that of type 1 diabetes for certain ethnic subgroups.9 Culturally grounded interventions can be made readily tailored and translated into real-world settings by utilizing collaborative, community-based participatory approaches. From a public health perspective, these approaches may offer the greatest likelihood for closing the obesity-related health disparities gap that currently exists among minority youth.10 Therefore, the purpose of this study was to examine the feasibility and preliminary effects of a culturally grounded, community-based T2D prevention program designed for obese Latino youth.
Methods
Study Design
The Every Little Step Counts–Diabetes Prevention Program (ELSC-DPP) was developed to address the growing need for improving obesity-related health among high-risk Latino youths. Investigators from a large academic research institution worked collaboratively with a local community clinic serving uninsured Latino families and a metropolitan YMCA to develop a diabetes prevention program for high-risk Latino adolescents. The collaboration was based upon a mutual respect for one another and a strong desire to integrate expertise and resources across institutions to create a unique and viable approach to serving a vulnerable population. The team functioned as equal partners throughout the development, implementation, and now dissemination process. This approach, known as community-based participatory research (CBPR),11 provided an opportunity for testing the culturally grounded intervention in a real-world setting using rigorous methods derived from a relevant theoretical approach. This pilot study used a nonrandomized pretest-posttest design to examine the feasibility and preliminary effects of the ELSC-DPP on insulin sensitivity and glucose tolerance among overweight/obese Latino adolescents. This design was selected in order to determine how well the intervention could be delivered, how well the intervention would be received by participants, and the potential effect size for reducing T2D risk in a future large-scale randomized controlled trial. This is considered an appropriate design for pilot studies.12 The study was approved by the Arizona State University Institutional Review Board, and participants and a parent or legal guardian provided written informed consent prior to any study procedure.
Participants
Eighteen Latino boys and girls were recruited by the community clinic through an established network of schools and school-based health centers. Potential participants were identified by the community partners and their referral sources for meeting the following general inclusion criteria: age 14 to 16 years, body mass index (BMI) percentile ≥85th for age and gender, and Latino ethnicity (child and parent self report). Youth were excluded if they participated in a formal weight management program during the previous 6 months prior to enrollment; were taking medications or diagnosed with a condition known to influence carbohydrate metabolism, physical activity, or cognitive function; or were type 2 diabetic upon screening. An equal number of males and females were recruited.
Procedures
Once general inclusion and exclusion criteria were met, potential participants and their parent/guardian were invited to the Arizona State University Clinical Research Unit for the administration of consent and baseline testing. Following an overnight fast, height (to the nearest 0.1 cm) and weight (to the nearest 0.1 kg) were measured in light clothing without shoes using a wall-mounted stadiometer and a medical balance beam scale. Standing waist circumference was measured at the level of the umbilicus to the nearest 0.1 cm after normal expiration using a Gulick II tape measure.
Lifestyle Behaviors
Physical activity was assessed by self-report using the 3-day Physical Activity Recall (3DPAR). The 3DPAR assesses a variety of typical physical activities among adolescents and is based upon 34 individual 30-minute time blocks of the 3 preceding days. Each day is broken up into 3 blocks (morning, afternoon, and evening), and participants are asked to recall specific activities as well as the corresponding intensity levels to those activities (light, moderate, hard or very hard). A list of 55 common activities is provided and pictorial representations are used to facilitate accurate assignment of intensity levels. The 3DPAR has been previously validated against accelerometry in adolescents.13
Dietary intake was measured using the Brief Dietary Assessment Tool for Hispanics.14 This screening tool was specifically designed using data from Mexican Americans participating in NHANES III to assess fruit, vegetable, and fat intake among Hispanics in community settings. The tool shows good reliability scores for both fruit and vegetable consumption (r = 0.64; P < 0.001) and fat intake (r = 0.85; P < 0.001) and may be well suited for assessing changes in these measures in response to interventions.
T2D Risk Factors
Insulin sensitivity and glucose tolerance were determined via a multiple sample oral glucose tolerance test (OGTT). Participants ingested 1.75 g of oral glucose solution per kilogram of body weight (to a maximum of 75 g). Blood samples were taken via antecubital vein catheter prior to glucose ingestion (fasting) and at 30, 60, 90, and 120 minutes post ingestion for measurement of plasma glucose (glucose oxidase, YSI INC., Yellow Sprigs, OH) and insulin (ELISA, ALPCO Diagnostics, Windham, NH). Total area under the curve (AUC) for plasma glucose and insulin during the OGTT were calculated by the trapezoidal method using 30-minute sampling time points.15 Insulin sensitivity was estimated by the whole-body insulin sensitivity index (WBISI) using plasma glucose and insulin concentrations as described by Matsuda and Defronzo16 using the following equation:
Exercise Capacity
On a separate day, cardiorespiratory fitness was estimated with a submaximal exercise test using a prediction equation developed and validated for obese youth.17 Briefly, resting heart rate was determined and participants walked on a treadmill at a self-selected testing speed at 0% grade for an initial 4-minute warm-up phase. The grade was then increased to 5%, and treadmill speed was maintained at the participant’s self-selected pace. After 4 minutes, heart rate was recorded using a heart rate monitor and participants were allowed to cool down. The following equation was used to estimate fitness:
Intervention
Upon completion of baseline testing, participants entered a 12-week lifestyle intervention that included weekly education sessions delivered in a group setting by bilingual/bicultural promotoras (health educators). The lifestyle education classes were held at the YMCA in a room that could accommodate the adolescents as well as their families. Familismo (familism) is a key cultural construct within the Latino community, so parental involvement was a requisite for participation and parents were encouraged to bring their other children to the education sessions in order to facilitate family health and encourage social support and modeling of behavior change for the entire family unit. In addition to familismo, other key cultural constructs that represented the foundation of the program included: Confianza, Respeto, y Personalismo (trust, respect, and personal interaction).18 The promotoras (N = 2) and research staff established rapport with participants and their families around these constructs in an effort to ground the program within the cultural context of the Latino community. The clinical partners have a long-standing relationship serving the Latino community, and a deep-structure understanding of the Latino culture has been essential to their success in working with the community.19
The 12-session intervention curriculum is presented in Table 1 and was guided by social cognitive theory20 using an adapted ecodevelopmental framework21 to support behavior change and T2D risk reduction. Health improvement rather than weight loss was the goal, and individual laboratory results provided the structure for teaching participants about improving obesity-related health risk factors such as insulin sensitivity and glucose tolerance. Classes were delivered using an interactive format where youth and families were encouraged to share their personal experiences, beliefs, successes, and challenges. Out of class activities such as grocery shopping with parents to prepare a healthy family meal were used to facilitate curriculum integration into day-to-day lifestyle changes. Throughout the program, youth and their families were asked reflection questions of how they incorporated information learned into their everyday life (eg, What did you do last week to improve how you feel about yourself?). Success was recognized and acknowledged and challenges were discussed with a focus on strategies to overcome barriers. At the conclusion of the program, youth were presented with a certificate of completion and were applauded for their efforts by the promotoras, families, peers, and the research team.
Table 1.
Intervention Outline
| Week | Lesson Title |
|---|---|
| 1 | Getting Started |
| 2 | Health Awareness |
| 3 | Roles and Responsibilities |
| 4 | Keep it Moving |
| 5 | How Sweet Are You? |
| 6 | Champions With Breakfast |
| 7 | Slim the Fat |
| 8 | Fast Food |
| 9 | Snack Attack |
| 10 | Stay Strong |
| 11 | Self-Esteem |
| 12 | Balancing Act |
In addition to lifestyle education classes, adolescents participated in three, 60-minute physical activity sessions per week. These sessions included individual and group activities that consisted of structured aerobic and resistance exercise and unstructured games. Heart rate was monitored on a weekly basis throughout the intervention with a target heart rate of 150 beats per minute for the majority of each 60-minute activity session. The physical activity curriculum was designed by the YMCA staff and tailored to the individual fitness levels of the participants. Although individual fitness goals were emphasized, participants supported and encouraged each other during group activities and games.
Upon completion of the 12-week intervention, participants returned to the Arizona State University Clinical Research Unit where baseline measures were reassessed as described previously.
Statistics
Descriptive characteristics are presented as means ± SE. Paired sample t tests were used to examine changes in height, weight, BMI, waist circumference, physical activity, dietary intake, and type 2 diabetes risk factors following the intervention. All data were analyzed using PASW Statistics version 18.0 with significance set at P < 0.05.
Results
Fifteen of the 18 adolescents enrolled at baseline completed the intervention. Two participants dropped out within the first week due to a lack of interest and another participant had a scheduling conflict with work that prevented regular attendance of the lifestyle education classes. No significant anthropometric or metabolic differences were observed between completers and non-completers with the exception of 2-hour glucose, which was lower in noncompleters (90.0 ± 16.0 vs 117.2 ± 4.8, P = 0.047). All subsequent analyses are performed on the 15 participants who completed the intervention unless otherwise noted.
Descriptive, anthropometric, fitness, activity, and nutrition data at baseline and following the intervention are presented in Table 2. Participants attended 91% of the intervention sessions where average heart rates were 150 ± 3.3 beats/min, and although weight did not change, significant decreases in BMI percentile, BMI z score, and waist circumference were observed following the intervention (all P < 0.05). In addition, cardiorespiratory fitness increased significantly, and although physical activity levels did not change significantly, by the end of the intervention 67% of the participants met the CDC recommendations of at least 60 minutes of moderate to vigorous physical activity/day (compared to 47% at baseline). Physical inactivity, as measured by total screen time (TV and computer), decreased nearly 47% from 178.6 ± 40.5 to 95.0 ± 26.4 minutes per day (P = 0.01). Significant reductions in reported servings of fat per day were observed, whereas no significant changes in servings of fruits and vegetables were reported (Table 3).
Table 2.
Descriptive Characteristicsa
| Measure | Pre | Post | Percent Change | P Value |
|---|---|---|---|---|
| Height (cm) | 165.8 ± 3.1 | 166.2 ± 3.2 | 0.24 | 0.05 |
| Weight (kg) | 90.6 ± 6.8 | 89.9 ± 7.2 | −0.001 | 0.44 |
| BMI (kg/m2) | 32.5 ± 1.6 | 32.0 ± 1.7 | −1.5 | 0.06 |
| BMI percentile (%) | 96.3 ± 1.1 | 95.0 ± 1.5 | −1.3 | 0.02 |
| BMI z score | 2.0 ± 0.1 | 1.9 ± 0.2 | −5.0 | 0.01 |
| Waist circumference (cm) | 107.0 ± 4.3 | 103.1 ± 5.0 | −3.6 | 0.02 |
Abbreviation: BMI, body mass index.
Data presented as means ± SE.
Table 3.
Changes in Fitness, Activity, and Nutritiona
| Measure | Pre | Post | Percent Change | P Value |
|---|---|---|---|---|
| VO2peak (L/min) | 2.8 ± 0.2 | 2.9 ± 0.2 | 3.6 | 0.01 |
| VO2peak (ml/kg/min) | 30.5 ± 0.9 | 32.1 ± 0.9 | 5.2 | 0.002 |
| MVPA (30 min blocks/day) | 2.3 ± 0.6 | 2.9 ± 0.5 | 26.1 | 0.32 |
| Physical inactivity (30 min blocks/day) | 15.7 ± 1.5 | 11.5 ± 0.6 | −26.8 | 0.002 |
| Screen time (30 min blocks/day) | 5.6 ± 1.3 | 3.0 ± 0.9 | −46.4 | 0.02 |
| Dietary fat (servings/day) | 3.3 ± 0.3 | 2.0 ± 0.2 | −39.4 | 0.001 |
| Fruits and vegetables (servings/day) | 2.9 ± 0.5 | 2.7 ± 0.4 | −6.9 | 0.72 |
Abbreviation: MVPA, moderate to vigorous physical activity.
Data presented as means ± SE.
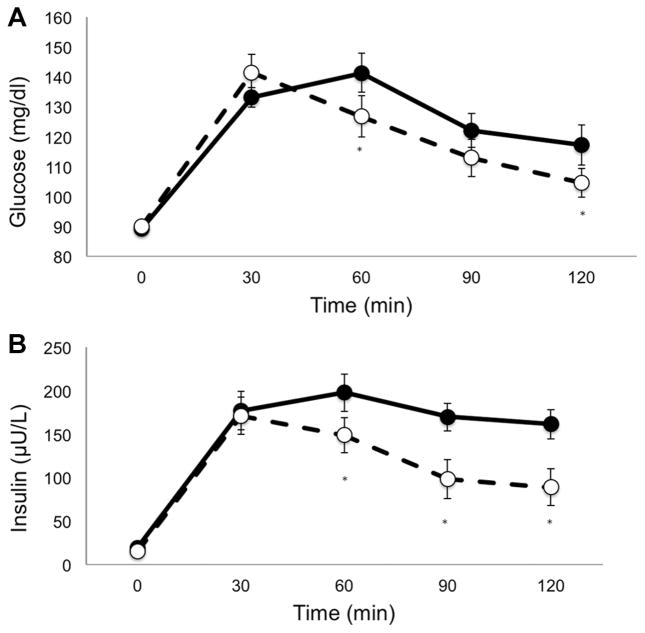
Figure 1A and B presents changes in glucose (A) and insulin (B) concentrations during the OGTT. Although fasting glucose did not change, a trend toward significance was noted for decreases in fasting insulin (P = 0.06). Significant decreases were observed in 2-hour glucose (10.8%), 2-hour insulin (23.6%), as well as AUC for glucose (16.2%) and insulin (25.5%), all P < 0.01. These improvements correspond to a 29.2% increase in insulin sensitivity as measured by the WBISI (2.4 ± 0.3 to 3.1 ± 0.3; P = 0.01). The only participant who presented with impaired glucose tolerance at baseline (2-hour glucose = 151 mg/dl) was no longer pre-diabetic following the intervention (2-hour glucose = 122 mg/dl).
Figure 1.
Glucose (A) and insulin (B) response to a 2-hour oral glucose tolerance test before (black circles and solid line) and after (open circles and dotted line) intervention.
*P < 0.05 compared to corresponding baseline value.
Discussion
Latino adolescents are disproportionately impacted by obesity and T2D, yet despite recent calls for action,10,22,23 very few culturally grounded diabetes prevention programs have been described in the literature for this population. This article describes a community-based participatory approach for developing and testing the feasibility of a 12-week lifestyle intervention and presents the preliminary efficacy of the intervention for improving T2D risk factors among obese Latino youth. The findings extend previous clinical studies in obese youth24,25 and support the translation of diabetes prevention programs for this population into community settings.
With the growing epidemic of obesity among children and adolescents, the Institute of Medicine has recently emphasized the importance of preventing obesity in young children.26 However, among children and adolescents who are already obese, targeting improvements in more proximal measures of diabetes risk such as reducing insulin resistance and/or improving glucose tolerance appear warranted.27 Insulin resistance, (ie, decreased insulin sensitivity), is thought to be one of the earliest pathophysiological processes of T2D.28 Insulin resistance prospectively predicts the development of T2D in adults,29 and adolescents with T2D are significantly more insulin resistant than obese controls.30 Therefore, increasing insulin sensitivity may be a key factor for preventing T2D in high-risk youth. Adolescents who participate in greater physical activity exhibit higher insulin sensitivity compared to more sedentary peers.31 Moreover, intervention studies support the utility of exercise to improve insulin sensitivity in obese adolescents.32,33 The amount of physical activity included in the ELSC intervention (ie, 60 minutes of moderate to vigorous physical activity 3 days/week) is a sufficient dose to improve insulin sensitivity in obese youth32 and may contribute to the observed health benefits.
Despite the improvement in insulin sensitivity, non-significant changes in weight and only small changes in BMI and BMI percentile were observed. However, significant reductions in waist circumference following the intervention were noted. Studies in adults suggest that exercise can result in significant reductions in waist circumference and/or abdominal obesity even in the absence of weight loss.34,35 Given the independent effect of abdominal adiposity on insulin resistance in Latino children36 and T2D in Latino adults,37 refocusing attention on reducing abdominal obesity to support health improvements rather than weight loss in this population may be prudent. As part of the lifestyle education curriculum, participants and their families were provided results of baseline laboratory testing in the context of T2D risk education. During the first session, families were informed that the goal of the ELSC program was to empower participants to improve health and reduce diabetes risk through healthy behaviors rather than weight loss. This message was reinforced throughout the program where adolescents and parents were encouraged to appreciate various aspects of health in a comprehensive manner.
Rather than promoting caloric restriction, the nutrition education sessions focused on the importance of eating breakfast, increasing fruit and vegetable consumption, reducing calories from fat and added sugar, portion control, and healthy snacking. Families were encouraged to set nutrition goals, share favorite recipes or foods, and the promotoras facilitated discussion about ways to exchange unhealthy ingredients and cooking methods for healthier options (eg, substituting with low fat cheese and skim milk, grilling chicken instead of frying it). In addition, as part of the roles and responsibilities session, adolescents were encouraged to shop for and prepare a healthy meal for their families as a way for them to develop a sense of responsibility for their own as well as their family’s health. This is an important skill to develop during adolescence as the transition to adulthood brings greater autonomy and responsibility for food preparation and subsequent eating behaviors.38
The study was guided by an expanded ecodevelopmental model, which recognizes the importance of critical life periods in terms of disease pathogenesis as well as prevention.21 The ELSC-DDP specifically targeted adolescence as a critical developmental period for implementing targeted diabetes prevention programs. The pubertal transition is associated with specific biological as well as behavioral changes that are directly linked to T2D. From a biological perspective, puberty is associated with a physiological insulin resistance that is thought to contribute to T2D among high-risk adolescents.39 From a behavioral perspective, adolescence is associated with a significant decline in physical activity, which is thought to be steepest between ages 13 and 1840 and may further contribute to T2D risk. In this expanded ecodevelopmental model, these factors fall within individual and organic level systems, but including multiple external systems in order to facilitate individual health is proposed to be a more efficacious strategy. The ELSC-DPP intervention included the contextual-ecological influences of family, community, and sociocultural factors to support individual-level changes in health behaviors and outcomes. Family support was encouraged through participation in the lifestyle education sessions that were delivered in the community, by the community using a culturally grounded approach. The preliminary efficacy of this intervention may be, in part, attributable to the coordinated inclusion of these multiple systems (cultural, community, family) under a unified health enhancement approach, and thus likely introduced synergistic effects in support of healthy behavior changes among the individual participants. Scholars from the National Institute of Minority Health and Health Disparities have encouraged researchers to employ translational, transformation, and transdiciplinary approaches in order to shift the paradigm in health disparities research.41 The ecodevelopmental model, which is operationalized through the ELSC-DPP intervention, is one such approach that holds promise in closing the health disparities gap among minority youth.
Translating diabetes prevention programs into the community has proven to be a successful model for reducing diabetes risk and improving health outcomes in adults.42 The Diabetes Prevention Program (DPP) established that intensive lifestyle intervention can prevent (or delay) the onset of T2D in high-risk adults.43 Although the DPP employed a rigorous scientific approach, it was extremely costly and not immediately translatable into real-world settings.44 Furthermore, while the trial included a large number of minority participants, the intervention itself was not culturally grounded to meet the specific needs of minority individuals or communities. Since the publication of the DPP results, several studies have successfully adapted the DPP curriculum for use in community settings, many of which target minority populations, and a few have been implemented using a community-based participatory approach.42 These studies support the hypothesis that culturally tailored diabetes prevention programs delivered in the community setting may provide the best opportunity for closing the diabetes-related health disparities gap. The ELSC-DPP extends previous adult models of implementing culturally grounded community-based diabetes prevention interventions to a group of high-risk Latino adolescents.
This program is somewhat unique to other community-based health programs for youth in that the delivery of the intervention was not in partnership with schools or the school system. Previous school-based obesity interventions have yielded modest effects on weight-related outcomes and very few have been successful in terms of reducing T2D risk factors in adolescents.45 Although schools may appear to be a logical venue for implementing diabetes prevention programs for children and adolescents, the limited success of previous studies and the economic challenges schools are facing suggest that alternative community-based strategies be tested. The ELSC-DPP was delivered in a YMCA located in a large municipal setting. The primary reason for partnering with the YMCA was the mutual interest in developing diabetes prevention programs for Latino youth in the collective community. However, from a public health perspective, the YMCA may be an ideal venue to deliver diabetes prevention programs on a large scale. It is estimated that ~70 million US households live within 3 miles of a YMCA and the YMCA reaches more than 10 million children and adolescents in over 10 000 US communities.46 From a policy perspective, the YMCA’s mission is to strengthen the communities it serves regardless of gender, income, faith, sexual orientation, or cultural background. Furthermore, the YMCA has proven to be a viable venue for translating the DPP curriculum into the community to prevent diabetes in high-risk adults.47 Taken together, these results suggest that culturally grounded diabetes prevention programs for Latino youth can be successfully delivered in the community setting through the YMCA.
The strength of this study includes the focus on a high-risk population of adolescents, a culturally grounded intervention developed in collaboration with the community for delivery in the community, and the inclusion of robust measures of T2D risk. Despite these strengths, there are limitations that are worthy of comment. First, the relatively small sample size may limit the power to detect smaller effect sizes, as well as limiting the generalizability of our findings. However, this was a feasibility study and the intervention was developed specifically to meet the needs of obese Latino adolescents in a defined community. While these findings may not be generalizable, this approach can and should be tested in other communities and populations, given a local adaptation to ground the intervention consistent with the culture of the local community. The lack of a randomized control design also limits the ability to draw definitive conclusions as to whether the results were uniquely attributable to the intervention relative to an alternate source of influence. Clearly it is important to maintain scientific rigor in order to conduct sound science, but in order for translational science to truly be transformative, approaches must be viable in real-world settings.48 It is challenging enough for scientists to establish the necessary rapport and trust to develop meaningful collaborations with vulnerable communities, and to expect these communities to agree to exclude up to half of their population from receiving potential health benefits (ie, randomized to a control or usual care group) may be unrealistic and do little to close the widening health disparities gap.48 Nevertheless, future studies should evaluate the efficacy of similar programs against a more traditional control or reference group.
In conclusion, these promising results suggest that a culturally grounded community-based lifestyle intervention program can reduce T2D risk factors among obese Latino adolescents. Translational approaches that include community collaboration and family involvement to improve robust individual health outcomes among high-risk youth should be tested in various populations. If successful, this approach may substantially reduce the overall burden of obesity-related disease in our society.
Acknowledgments
We are grateful to the families who participated in the ELSC-DPP and the promotoras (Saray Gordillo, Elva Madrid, and Elvia Madrid) who served as a critical link between the participants and the research team. We thank the administrative directors of our community partners (Janice Ertl, RN, MHSA, Virginia G. Piper Medical & Dental Clinic, and Jeff Myers, Lincoln Family YMCA) for their support and encouragement and Cecilia Chapman, RD, CDE, for her help developing the program. Dr Shaibi completed this work as part of an Early Career Faculty Fellowship in health disparities research with the ASU Southwest Interdisciplinary Research Center an Exploratory Center of Excellence for Health Disparities Research and Training. The project was funded by the ASU Southwest Interdisciplinary Research Center through a grant from the National Institutes of Health, National Center on Minority Health and Health Disparities (P20MD002316).
References
- 1.Lee JM, Okumura MJ, Davis MM, Herman WH, Gurney JG. Prevalence and determinants of insulin resistance among U.S. adolescents: a population-based study. Diabetes Care. 2006;29(11):2427–2432. doi: 10.2337/dc06-0709. [DOI] [PubMed] [Google Scholar]
- 2.Johnson WD, Kroon JJM, Greenway FL, Bouchard C, Ryan D, Katzmarzyk PT. Prevalence of risk factors for metabolic syndrome in adolescents: National Health and Nutrition Examination Survey (NHANES), 2001–2006. Arch Pediatr Adolesc Med. 2009;163(4):371–377. doi: 10.1001/archpediatrics.2009.3. [DOI] [PubMed] [Google Scholar]
- 3.Goran MI, Bergman RN, Avila Q, et al. Impaired glucose tolerance and reduced β-cell function in overweight Latino children with a positive family history for type 2 diabetes. J Clin Endocrinol Metab. 2004;89(1):207–212. doi: 10.1210/jc.2003-031402. [DOI] [PubMed] [Google Scholar]
- 4.Cruz ML, Weigensberg MJ, Huang TT, Ball G, Shaibi GQ, Goran MI. The metabolic syndrome in overweight Hispanic youth and the role of insulin sensitivity. J Clin Endocrinol Metab. 2004;89(1):108–113. doi: 10.1210/jc.2003-031188. [DOI] [PubMed] [Google Scholar]
- 5.Narayan KM, Boyle JP, Thompson TJ, Sorensen SW, Williamson DF. Lifetime risk for diabetes mellitus in the United States. JAMA. 2003;290(14):1884–1890. doi: 10.1001/jama.290.14.1884. [DOI] [PubMed] [Google Scholar]
- 6.Gillies CL, Abrams KR, Lambert PC, et al. Pharmacological and lifestyle interventions to prevent or delay type 2 diabetes in people with impaired glucose tolerance: systematic review and meta-analysis. BMJ. 2007;334(7588):299. doi: 10.1136/bmj.39063.689375.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang TTK, Goran MI. Prevention of type 2 diabetes in young people: a theoretical perspective. Pediatr Diabetes. 2003;4:38–56. doi: 10.1034/j.1399-5448.2003.00022.x. [DOI] [PubMed] [Google Scholar]
- 8.Franks PW, Huang TT, Ball G. Lifestyle intervention for type 2 diabetes risk reduction:using the diabetes prevention program to inform new directions in pediatric research. Can J Diabetes. 2007;31(3):242–251. [Google Scholar]
- 9.The Writing Group for the SEARCH for Diabetes in Youth Study Group. Incidence of diabetes in youth in the United States. JAMA. 2007;297(24):2716–2724. doi: 10.1001/jama.297.24.2716. [DOI] [PubMed] [Google Scholar]
- 10.Vivian EM. Strategies and considerations for community-based participatory research in the prevention of type 2 diabetes in youth. Diabetes Spectr. 2010;23(4):213–215. [Google Scholar]
- 11.Minkler M, Blackwell AG, Thompson M, Tamir H. Community-based participatory research: implications for public health funding. Am J Public Health. 2003;93(8):1210–1213. doi: 10.2105/ajph.93.8.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thabane L, Ma J, Chu R, et al. A tutorial on pilot studies: the what, why and how. BMC Med Res Methodol. 2010;10(1):1. doi: 10.1186/1471-2288-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pate RR, Ross R, Dowda M, Trost SG, Sirard J. Validation of a 3-day physical activity recall instrument in female youth. Pediatr Exerc Sci. 2003;15(3):257–265. [Google Scholar]
- 14.Wakimoto P, Block G, Mandel S, Medina N. Development and reliability of brief dietary assessment tools for Hispanics. Prev Chronic Dis. 2006;3(3):A95. [PMC free article] [PubMed] [Google Scholar]
- 15.Matthews JN, Altman DG, Campbell MJ, Royston P. Analysis of serial measurements in medical research. BMJ. 1990;300(6719):230–235. doi: 10.1136/bmj.300.6719.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22(9):1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 17.Nemeth BA, Carrel AL, Eickhoff J, Clark RR, Peterson SE, Allen DB. Submaximal treadmill test predicts VO2max in overweight children. J Pediatr. 2009;154(5):677–681. doi: 10.1016/j.jpeds.2008.11.032. [DOI] [PubMed] [Google Scholar]
- 18.Castro FG, Alarcon EH. Integrating cultural variables into drug abuse prevention and treatment with racial/ethnic minorities. J Drug Issues. 2002;32(3):783–811. [Google Scholar]
- 19.Resnicow K, Soler R, Braithwait RL, Ahluwalia JS, Butler J. Cultural sensitivity in substance abuse prevention. J Comm Psychol. 2000;28:271–290. [Google Scholar]
- 20.Bandura A. Social Cognitive Theory. Vol. 6. Greenwich, CT: JAI Press LTD; 1989. [Google Scholar]
- 21.Castro FG, Shaibi GQ, Boehm-Smith E. Ecodevelopmental contexts for preventing type 2 diabetes in Latino and other racial/ethnic minority populations. J Behav Med. 2009;32(1):89–105. doi: 10.1007/s10865-008-9194-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Branscum P, Sharma M. A systematic analysis of childhood obesity prevention interventions targeting Hispanic children: lessons learned from the previous decade. Obes Rev. 2011;12(5):e151–e158. doi: 10.1111/j.1467-789X.2010.00809.x. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Tussing L. Culturally appropriate approaches are needed to reduce ethnic disparity in childhood obesity. J Am Diet Assoc. 2004;104(11):1664–1666. doi: 10.1016/j.jada.2004.08.035. [DOI] [PubMed] [Google Scholar]
- 24.Savoye M, Shaw M, Dziura J, et al. Effects of a weight management program on body composition and metabolic parameters in overweight children: a randomized controlled trial. JAMA. 2007;297(24):2697–2704. doi: 10.1001/jama.297.24.2697. [DOI] [PubMed] [Google Scholar]
- 25.van der Heijden GJ, Toffolo G, Manesso E, Sauer PJ, Sunehag AL. Aerobic exercise increases peripheral and hepatic insulin sensitivity in sedentary adolescents. J Clin Endocrinol Metab. 2009;94(11):4292–4299. doi: 10.1210/jc.2009-1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Birch L, Parker L, Burns A. Early Childhood Obesity Prevention Policies. Washington, DC: National Academies Press; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cruz ML, Shaibi GQ, Weigensberg MJ, Spruijt-Metz D, Ball GDC, Goran MI. Pediatric obesity and insulin resistance: chronic disease risk and implications for treatment and prevention beyond body weight modification. Annu Review Nutr. 2005;25(1):435–468. doi: 10.1146/annurev.nutr.25.050304.092625. [DOI] [PubMed] [Google Scholar]
- 28.Ferrannini E. Insulin resistance versus insulin deficiency in non-insulin-dependent diabetes mellitus: problems and prospects. Endocr Rev. 1998;19(4):477–490. doi: 10.1210/edrv.19.4.0336. [DOI] [PubMed] [Google Scholar]
- 29.Weyer C, Tataranni PA, Bogardus C, Pratley RE. Insulin resistance and insulin secretory dysfunction are independent predictors of worsening of glucose tolerance during each stage of type 2 diabetes development. Diabetes Care. 2001;24(1):89–94. doi: 10.2337/diacare.24.1.89. [DOI] [PubMed] [Google Scholar]
- 30.Elder DA, Prigeon RL, Wadwa RP, Dolan LM, D’Alessio DA. β-cell function, insulin sensitivity, and glucose tolerance in obese diabetic and nondiabetic adolescents and young adults. J Clin Endocrinol Metab. 2006;91(1):185–191. doi: 10.1210/jc.2005-0853. [DOI] [PubMed] [Google Scholar]
- 31.Imperatore G, Cheng YJ, Williams DE, Fulton J, Gregg EW. Physical activity, cardiovascular fitness, and insulin sensitivity among U.S. adolescents: the National Health and Nutrition Examination Survey, 1999–2002. Diabetes Care. 2006;29(7):1567–1572. doi: 10.2337/dc06-0426. [DOI] [PubMed] [Google Scholar]
- 32.Bell LM, Watts K, Siafarikas A, et al. Exercise alone reduces insulin resistance in obese children independently of changes in body composition. J Clin Endocrinol Metab. 2007;92(11):4230–4235. doi: 10.1210/jc.2007-0779. [DOI] [PubMed] [Google Scholar]
- 33.Shaibi GQ, Cruz ML, Ball GD, et al. Effects of resistance training on insulin sensitivity in overweight Latino adolescent males. Med Sci Sports Exerc. 2006;38(7):1208–1215. doi: 10.1249/01.mss.0000227304.88406.0f. [DOI] [PubMed] [Google Scholar]
- 34.Church TS, Martin CK, Thompson AM, Earnest CP, Mikus CR, Blair SN. Changes in weight, waist circumference and compensatory responses with different doses of exercise among sedentary, overweight postmenopausal women. PloS one. 2009;4(2):e4515. doi: 10.1371/journal.pone.0004515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giannopoulou I, Ploutz-Snyder LL, Carhart R, et al. Exercise is required for visceral fat loss in postmenopausal women with type 2 diabetes. J Clin Endocrinol Metab. 2005;90(3):1511–1518. doi: 10.1210/jc.2004-1782. [DOI] [PubMed] [Google Scholar]
- 36.Cruz ML, Bergman RN, Goran MI. Unique effect of visceral fat on insulin sensitivity in obese Hispanic children with a family history of type 2 diabetes. Diabetes Care. 2002;25(9):1631–1636. doi: 10.2337/diacare.25.9.1631. [DOI] [PubMed] [Google Scholar]
- 37.Wei M, Gaskill SP, Haffner SM, Stern MP. Waist circumference as the best predictor of noninsulin dependent diabetes mellitus (NIDDM) compared to body mass index, waist/hip ratio and other anthropometric measurements in Mexican Americans—a 7-year prospective study. Obes Res. 1997;5(1):16–23. doi: 10.1002/j.1550-8528.1997.tb00278.x. [DOI] [PubMed] [Google Scholar]
- 38.Bassett R, Chapman GE, Beagan BL. Autonomy and control: the co-construction of adolescent food choice. Appetite. 2008;50(2–3):325–332. doi: 10.1016/j.appet.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 39.Goran MI, Gower BA. Longitudinal study on pubertal insulin resistance. Diabetes. 2001;50(11):2444–2450. doi: 10.2337/diabetes.50.11.2444. [DOI] [PubMed] [Google Scholar]
- 40.Sallis JF. Age-related decline in physical activity: a synthesis of human and animal studies. Med Sci Sports Exerc. 2000;32(9):1598–1600. doi: 10.1097/00005768-200009000-00012. [DOI] [PubMed] [Google Scholar]
- 41.Dankwa-Mullan I, Rhee KB, Stoff DM, et al. Moving toward paradigm-shifting research in health disparities through translational, transformational, and transdisciplinary approaches. Am J Public Health. 2010;100(Suppl 1):S19–S24. doi: 10.2105/AJPH.2009.189167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jackson L. Translating the Diabetes Prevention Program into practice: a review of community interventions. Diabetes Educ. 2009;35(2):309–320. doi: 10.1177/0145721708330153. [DOI] [PubMed] [Google Scholar]
- 43.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ackermann RT, Marrero DG. Adapting the Diabetes Prevention Program lifestyle intervention for delivery in the community: the YMCA model. Diabetes Educ. 2007;33(1):69–78. doi: 10.1177/0145721706297743. [DOI] [PubMed] [Google Scholar]
- 45.Gittelsohn J, Kumar MB. Preventing childhood obesity and diabetes: is it time to move out of the school? Pediatr Diabetes. 2007;8 (Suppl 9):55–69. doi: 10.1111/j.1399-5448.2007.00333.x. [DOI] [PubMed] [Google Scholar]
- 46.Adamson K, Shepard D, Easton A, ESJ The YMCA/Steps Community Collaboratives, 2004–2008. Prev Chronic Dis. 2009;6(3):1–5. [PMC free article] [PubMed] [Google Scholar]
- 47.Ackermann RT, Finch EA, Brizendine E, Zhou H, Marrero DG. Translating the Diabetes Prevention Program into the community: The DEPLOY pilot study. Am J Prev Med. 2008;35(4):357–363. doi: 10.1016/j.amepre.2008.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kessler R, Glasgow RE. A proposal to speed translation of health-care research into practice: dramatic change is needed. Am J Prev Med. 2011;40(6):637–644. doi: 10.1016/j.amepre.2011.02.023. [DOI] [PubMed] [Google Scholar]