Abstract
We tested the hypothesis that the sensory-motor characteristics of aerodigestive reflexes are dependent on stimulus type and volumes, sleep or awake states, and maturation. Thirteen neonates were studied at 33.6 ± 0.5 wk (time 1) and 37.3 ± 0.5 wk (time 2) postmenstrual age using multimodal provocative esophageal manometry concurrent with video polysomnography. Effects of graded volumes (399 infusions at time 1, 430 infusions at time 2) of midesophageal stimulation with air, water, and apple juice on the sensory thresholds and recruitment frequency of upper esophageal sphincter (UES), esophageal body, and lower esophageal sphincter (LES) reflexes were investigated during sleep and awake states. Sensory thresholds for aerodigestive reflexes between maturational stages were similar. Increased frequency recruitment of UES contractile reflex, LES relaxation reflex, and peristaltic reflexes were noted at time 2 (all, P < 0.05). Graded stimulus-response relationships were evident at time 1 and time 2 during awake and sleep states (P < 0.05). Secondary peristalsis vs. esophago-deglutition response proportions during sleep at time 1 vs. time 2 (P = 0.001) and awake vs. sleep at time 2 (P = 0.02) were distinct. We concluded that sensory-motor effects of esophageal mechanosensitivity, osmosensitivity, and chemosensitivity are advanced in sleep with maturation. Sleep further modulates the frequency recruitment and the type of aerodigestive reflexes.
Keywords: sleep, upper esophageal sphincter, lower esophageal sphincter, peristaltic reflex
sleep is both a crucial and vulnerable physiological state for the developing infant. Although it is essential for conservation of energy and growth, the aerodigestive tract also becomes increasingly susceptible to both retrograde and anterograde aspiration during periods of sleep. Aerodigestive adaptation to these provocations must be maintained; however, the effects of such esophageal provocation during sleep are unclear. Compromise of aerodigestive functions may have serious consequences such as apparent life-threatening events (ALTE) or sudden infant death syndrome (SIDS) (1). It is common practice for infants who have experienced such events to undergo evaluation for esophageal and sleep pathology, as there is often a presumed causal connection between ALTE, SIDS, and aerodigestive pathology with respect to aspiration. These infants are often treated empirically for gastroesophageal reflux disease (GERD) and associated sleep disturbance, using treatments such as caffeine, positional changes, modifications of milk type or density, changes in feeding volumes or feeding methods, medical therapy, or even surgical intervention (2, 6, 25, 36). These treatments have aerodigestive consequences, may be expensive, and have uncertain utility. Unfortunately, more effective treatments are unavailable at this time, as there is little information on the aerodigestive defensive reflexes that facilitate stimulus clearance in relation to esophageal provocation in preterm infants, either in health or disease. The effects of sleep on the modulation of aerodigestive defense mechanisms are also unknown.
Sleep is often interrupted by environmental or visceral stimuli with variable physiological and pathological consequences (35). Recently validated methods in human premature neonates (11, 15–17, 30) allow for definition of the vagally mediated upper esophageal sphincter (UES) contractile reflex, esophageal peristaltic reflexes, and lower esophageal sphincter (LES) relaxation response evoked upon midesophageal provocation (15, 16, 19). This study was designed to clarify aerodigestive reflex physiology with regard to the influence of sleep state on the response frequency and magnitude of upstream (UES) and downstream (esophageal and LES) responses in infants during maturation.
Our aims were to test the hypothesis that esophageal stimulus-provoked aerodigestive reflex characteristics are dependent on: 1) stimulus type (gas or liquids), 2) stimulus volumes, 3) state of activity (awake, active-sleep, or quiet-sleep states), and 4) infant's maturation. The following aerodigestive reflexes were the focus of the investigation: UES contractile reflex, esophageal peristaltic reflexes, and LES relaxation reflex. This was accomplished by characterizing the esophageal responses to graded stimulus volumes (air and liquids) during the states of activity and sleep determined using video- polysomnography (PSG) with concurrent esophageal manometry in premature infants. The rationale for the targeted use of graded volumes of air, water, and apple juice (acidic) as provoking stimuli stems from the fact that the nature of esophageal stimuli can be either gastric contents or a swallowed bolus, the composition of which can have varying physico-chemical characteristics.
MATERIALS AND METHODS
Subjects.
Thirteen enterally fed preterm neonates (6 males, 23.7–32.0 wk gestation) were studied longitudinally, twice (26 studies) at 33.6 ± 0.5 and 37.3 ± 0.5 wk postmenstrual age (PMA). Subjects were studied after recovery from neonatal transition and respiratory distress and were on a stable phase of feeding and growth. All subjects were evaluated by the principal investigator (SRJ) and the attending neonatologist and were deemed healthy at study. None of the subjects had a presumed or proven clinical diagnosis of GER, and none were receiving prokinetics, acid-suppressive therapy, or xanthines at the time of study or discharge. Subjects with perinatal asphyxia, birth defects, and genetic syndromes were excluded.
The study procedures were approved by the ethics committee at the Institutional Research Review Board at the Nationwide Children's Hospital Research Institute, Columbus, Ohio. The study protocol conforms to the guidelines of the IRB policy and the health insurance portability and accountably acts (HIPAA). Informed consents and HIPAA authorization were obtained from parents before study.
Manometry methods.
Esophageal provocations were multimodal, and recordings were made using esophageal manometry methods in neonates, as described by us before (15, 16). Briefly, the catheter assembly (Dentsleeve International; Mui Scientific, Ontario, Canada) was connected to the pneumohydraulic micromanometric water perfusion system via the resistors, pressure transducers (TNF-R disposable pressure transducers), and amplifiers (solar modules, Solar 2; MMS Medical Instruments, Dover, NH). The esophageal manometry catheter assembly with dual sleeves and four side-ports recording from pharynx, proximal, middle, and distal esophageal loci and a terminal gastric recording port was used. The water perfusion rate was 0.02 ml/min per port for esophageal ports, 0.01 ml/min per port for the pharyngeal port, and 0.04 ml/min per port for the sleeves. The catheter was passed nasally in the supine lying unsedated neonate with the transducers at the level of the subject's esophagus. Nasogastric feeding tubes were always removed at the time of study in all subjects, as has been our study protocol. Once our manometry catheter is in place and well secured, we did not manipulate the manometry catheter during the study. Infants were allowed to adapt to catheter placement before the experimental protocol was carried out.
Manometric experimental protocol.
Pharyngo esophageal manometry catheter pull-through was performed first, and the locations of UES and LES high-pressure zones were verified, in addition to esophageal body and peristaltic waveforms. The catheter was well secured throughout the study. Continuous data acquisition and analysis were performed during manometric study based on waveform characteristics, as previously defined (15, 16, 21). The high-pressure zones of the LES and UES were identified during manometric pull through by the consistent increase in pressure >5.0 mmHg above the baseline for at least 15 s, in addition to the changes in pressure with respiration (15, 16, 21). Fifteen minutes after adaptation to catheter placement, upstream and downstream responses to graded volumes of abrupt midesophageal infusions (graded stimuli, 0.1–5.0 ml, air, sterile water, and apple juice) were evaluated. We provided esophageal stimulus in the same manner, i.e., infusions were given abruptly by giving a measured volume rapidly. The rationale for this practice is that volumes and flow of gastroesophageal refluxate vary and that we are only simulating GE reflux by testing the effects of variable graded volumes of mechanosensitive (air infusions), chemosensitive (apple juice infusions), and osmosensitive (sterile water infusions) media on the ability to evoke aerodigestive reflexes. Specifically, secondary peristalsis (SP), esophago-deglutition responses (EDR), UES contractile reflex, and LES relaxation reflex were evaluated for their respective sensory-motor characteristics during sleep and awake states. Infusions were given sequentially in the same order regardless of the activity state. Manometric data examiners were blinded to sleep state, and all esophageal provocations were performed by the same investigator (SRJ).
Video PSG methods.
Infant sleep study was performed and scored by accepted EEG methods. The Grass sleep system (Astro-Med; Grass Technologies, West Warwick, RI) and the TWin PSG Clinical Software were used. A standard infant montage including C3, C4, CZ, O1, O2, A1, and A2 gold-plated electrodes were used. EOG electrodes and chin EMG electrodes were applied, in addition to the EKG, thoracic, and abdominal respitrace belts. PSG leads were first placed, and infants were allowed to adapt. The sleep stages and arousals were scored per the established guidelines for neonates (10, 31, 32). PSG, respiratory inductance PSG (Respitrace; Viasys, Conshohocken, PA), heart rate, and pulse oximetry were synchronized and recorded concurrent with manometry. The computer clocks recording PSG and manometry were synchronized to the millisecond before start of the study and verified at the end of the study.
PSG data analysis.
The polysomnograms were initially scored by a certified PSG technician and further verified and confirmed by sleep board-certified pediatric polysomnographers (MS) using a priori criteria and were classified into different sleep stages and arousals (10). The scorers commented on the sleep stage during a point of interest (e.g., onset of stimulus or sham events); however, the scorers were blinded to the nature of stimulus or the type of the manometric event. Sham events were also included, during which no stimulus was given. Infant sleep stages were separated per the standard criteria into active and quiet sleep and awake states. EEG sleep state was scored 10 s before the stimulus or spontaneous manometric event and was compared with that during and 10 s after the manometric event. Per the infant scoring rules, sleep should be present for >30 s before attempting to score an arousal; in addition, >10 s of uninterrupted sleep is required between cortical arousals (31).
Manometry data analysis.
Manometry data analytical characteristics have been described before (11, 15, 16, 21, 30) and are briefly defined as follows. 1) Resting UES pressure was measured as an average of five UES pressure measurements at end expiration observed before stimulus or spontaneous swallow. 2) Response onset to UES contractile reflex is defined as the time taken from the onset of stimulus for an increase in UES pressure of at least 4 mmHg above baseline. 3) Maximum UES contraction pressure was taken at the maximum pressure reached after onset of UES contraction. 4) UES contractile reflex duration is measured from the onset of UES contraction, to the peak of UES contractile reflex. 5) EDR was defined as a deglutition response to esophageal stimulation, which begins with onset of the pharyngeal waveform associated with UES relaxation and propagates into the proximal, middle, and distal esophageal segments and is accompanied by LES relaxation (19). 6) SP occurs in response to midesophageal provocation and is defined as the propagation of waveforms distally from the proximal, middle, and distal esophageal segments in the absence of pharyngeal waveform and UES relaxation. The onset of proximal esophageal upstroke from the stimulus onset defines the response latency for SP (19). 7) Response onset to peristaltic reflex was taken from onset of stimulus to the onset of EDR or SP. 8) Threshold volume was determined by the presence of a >50% response to an infusion and >50% response rate at subsequent increments.
Statistical analysis.
Mixed statistical models were used to analyze the data at time 1 and time 2. Frequency of reflex responses (i.e., UES contractile reflex, LES relaxation reflex, and peristaltic reflex) were detected as nominal response, and the proportion of responses were compared using χ2 test. The relationship between graded stimulus volumes and binary outcomes (nominal responses) for each reflex response (i.e., UES contractile reflex, LES relaxation reflex, and peristaltic reflex) were tested by multiple logistic regression models at each maturational stage, adjusting for sleep states (awake and sleep) and media effects (air and liquids). Because repeated responses were obtained from the same neonates at each study, a generalized estimating equation approach was used to test for differences in volume-response relationships. These models include a random subject effect to account for the correlation of measures within subject and also nested effects to model the appropriate variance-covariance structure. The odds ratio, 95% confidence intervals, and P values were reported to predict the likelihood of the specific reflex. Data were presented as means ± SE or (median, range) or percentage, unless stated otherwise. P < 0.05 was considered significant. All tests were performed using SAS (version 9.2; SAS Institute, Cary, NC).
RESULTS
Participant characteristics.
At time 1 evaluation, weight was 1.6 ± 0.2 kg, length was 41.4 ± 1.2 cm, and head circumference was 29 ± 0.6 cm. At time 2 study, weight was 2.3 ± 0.2 kg, length was 44.8 ± 1.1 cm, and head circumference was 32.1 ± 0.4 cm. Growth was appropriate at time 1 and time 2 (P = 0.0002 for all growth comparisons). All were receiving nutrition via nasogastric tube at time 1, whereas, at time 2, 12 out of 13 (92.3%) infants were receiving exclusive oral feeds, and 1 infant was transitioning to oral feeds (feeding methods at time 1 vs. time 2, P < 0.0001).
Characteristics of esophageal stimulus and sensory thresholds.
Active and quiet sleep states were combined into one sleep category because no difference was noted between these two states (active vs. quiet, respectively) for the frequency of UES contractile reflex (49.7% vs. 42.5% at time 1, P = 0.2; 56.3% vs. 53.4% at time 2, P = 0.6), the frequency of LES relaxation reflex (25.6% vs. 31.4% at time 1, P = 0.3; 41.5% vs. 48.3% at time 2, P = 0.2), or the frequency of peristaltic reflex (54.5% vs. 50.8% at time 1, P = 0.5; 61.5% vs. 63.0% at time 2, P = 0.8).
Out of 399 infusion stimuli (153 air, 122 water, 124 apple juice) at time 1, 57 infusions (14.3%) were given during awake and 342 infusions (85.7%) were given during sleep. Out of 430 infusions (172 air, 128 water, 130 apple juice) given at time 2, 42 infusions (9.8%) were given during awake and 388 infusions (90.2%) were given during sleep. Esophageal stimulus characteristics were similar at time 1 and time 2 (Table 1), and threshold volumes to evoke any specific reflex were also similar (Table 2). Only 36 out of 342 esophageal stimuli (10.5%) given during sleep at time 1 and only 20 out of 388 stimuli (5.2%) given during sleep at time 2 resulted in arousals (arousals, time 1 vs. time 2, P = 0.008).
Table 1.
Characteristics of provoking esophageal stimulus
| Time-1 |
Time-2 |
|||
|---|---|---|---|---|
| Characteristics | Awake | Sleep | Awake | Sleep |
| Infusion duration, s | 1.7 ± 0.2 | 1.8 ± 0.1 | 1.7 ± 0.2 | 1.6 ± 0.1 |
| Flow rate, ml/s | 1.0 ± 0.2 | 1.0 ± 0.1 | 1.3 ± 0.2 | 1.2 ± 0.1 |
| Proportion of stimuli, air: water: apple juice, N | 24:16:17 | 129:106:107 | 18:11:13 | 154:117:117 |
Values are means ± SE. The characteristics are similar at both time points.
Table 2.
Stimulus threshold volume (ml) that evoked specific esophageal reflexes
|
Time 1, ml |
Time 2, ml |
|||||
|---|---|---|---|---|---|---|
| Reflex | Air | Water | Apple Juice | Air | Water | Apple Juice |
| UES contractile reflex | 0.5 (0.1–1) | 0.5 (0.1–0.5) | 0.5 (0.3–0.75) | 0.3 (0.1–1) | 0.5 (0.3–0.5) | 0.5 (0.1–0.5) |
| LES relaxation reflex | 0.5 (0.1–0.5) | 0.1 (0.1–0.5) | 0.5 (0.5–0.5) | 0.5 (0.1–1) | 0.5 (0.1–0.625) | 0.5 (0.5–0.875) |
| Peristaltic reflex | 0.5 (0.1–0.5) | 0.1 (0.1–0.5) | 0.5 (0.4–0.5) | 0.1 (0.1–0.5) | 0.5 (0.1–0.5) | 0.5 (0.5–0.5) |
Values are medians (interquartile range). The characteristics are similar at both time points. UES, upper esophageal sphincter; LES, lower esophageal sphincter.
Testing the effect of maturation and sleep on recruitment frequency of esophageal reflexes.
Any one or combination of reflexes, i.e., UES contractile reflex, LES relaxation reflex, or peristaltic reflex, was recognized as a composite response. The recruitment frequency of composite response was 80.3% at time 1 study and 79.5% at time 2 study (P = 0.8). The recruitment frequency of specific reflexes in awake and sleep states is shown (Table 3). In awake state, significant maturational effect on the frequency recruitment was noted only for UES contractile reflex but not with LES relaxation reflex or peristaltic reflex. In contrast, during sleep, trends for increased recruitment of UES contractile reflex, LES relaxation reflex, and peristaltic reflex were noted with older age.
Table 3.
Effect of air or liquid stimulus on frequency recruitment (%) of esophageal reflexes during awake vs. sleep states across maturation
| Awake |
Sleep |
|||||
|---|---|---|---|---|---|---|
| Frequency, % | Time 1 | Time 2 | P Value | Time 1 | Time 2 | P Value |
| UES contractile reflex | ||||||
| Air | 20.8*† | 64.7 | 0.005 | 54.2* | 58.2 | 0.5 |
| Liquid | 78.1† | 33.3 | 0.001 | 42.5 | 53.2 | 0.03 |
| LES relaxation reflex | ||||||
| Air | 42.9 | 23.5† | 0.2 | 33.6 | 51.1 | 0.005 |
| Liquid | 43.3 | 28.6 | 0.3 | 26.3 | 41.7 | 0.0009 |
| Peristaltic reflex | ||||||
| Air | 58.3 | 61.1 | 0.8 | 55.1 | 68.0 | 0.03 |
| Liquid | 53.1 | 54.2 | 0.9 | 50.7 | 58.6 | 0.1 |
Values are expressed as percentages;
P < 0.05 for comparison between air and liquids at identical maturational stage;
P < 0.05 for comparison between awake and sleep stage at identical maturational stage.
Testing the relationship between graded stimuli and recruitment of esophageal reflexes.
Examples highlighting the effect of graded midesophageal stimuli on esophagus, UES, and LES in awake state (Fig. 1) and sleep state (Fig. 2) are shown. To test the effect of graded stimulus volumes on frequency recruitment of composite reflex response during sleep and awake states at time 1 and time 2, stimulus volume-response relationships were studied, and significance was noted at both time 1 and time 2 for both awake and sleep states (all P < 0.05, Fig. 3).
Fig. 1.

Concurrent esophageal motility and polysomnography in awake state. Esophageal motility recordings (bottom) concurrent with polysomnography (top) monitoring is shown. The specific state in this figure is awake state. Specifically, the effects of graded (0.1 ml, 1.0 ml, and 2.0 ml) midesophageal liquid infusions on the ability to activate esophageal reflexes are shown. During the awake state, note the absence of any esophageal motor response at 0.1 ml and the presence of primary peristalsis at 1.0-ml and 2.0-ml infusion volumes. The arrows depict direction of propagation of waveforms. UES, upper esophageal sphincter; LES, lower esophageal sphincter.
Fig. 2.
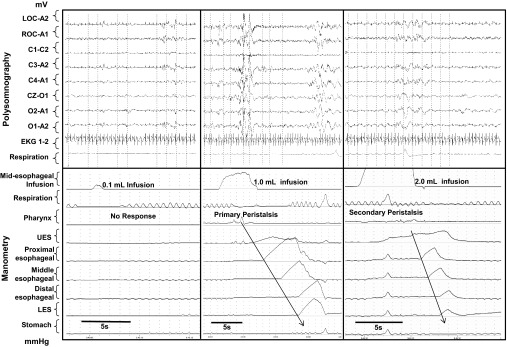
Concurrent esophageal motility and polysomnography in sleep state. Esophageal motility recordings (bottom) concurrent with polysomnography (top) monitoring is shown. The specific state in this figure is sleep state. Specifically, the effects of graded (0.1 ml, 1.0 ml, and 2.0 ml) midesophageal liquid infusions on the ability to activate esophageal reflexes are shown. During the sleep state, note the absence of any esophageal motor response at 0.1-ml, presence of primary peristalsis at 1.0-ml, and presence of secondary peristalsis and UES contractile response at 2.0-ml infusion volumes. The arrows depict direction of propagation of waveforms.
Fig. 3.
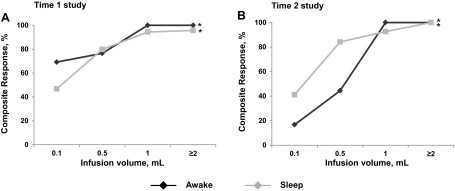
Stimulus volumes vs. esophageal reflex response relationship during awake vs. sleep states and at time 1 and time 2 phases of maturation. Recruitment of composite reflex responses with graded increments of esophageal stimuli is increasing in both awake and sleep states and also at both maturational stages. Volume-response relationship was evident at both awake and sleep stages (*P < 0.05).
Next, we evaluated the effects of 1) each stimulus media (air, water, apple juice), 2) graded volumes, 3) maturation, and 4) awake or sleep states on the recruitment of specific reflexes (UES contractile reflex, LES relaxation reflex, peristaltic reflex) using multiple logistic regression model and GEE methods (Table 4). Response frequency (UES contractile reflex, LES relaxation reflex, peristaltic reflex) was variable in awake state at both time 1 and time 2. In contrast, the probability of increased recruitment of the studied reflexes with incremental infusion volumes improved significantly during sleep state with maturation.
Table 4.
Graded stimulus volume-esophageal reflex response relationships across maturation and during sleep vs. awake states
| Awake - Time 1 |
Awake - Time 2 |
Sleep - Time 1 |
Sleep - Time 2 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Reflex | Media | Odds Ratio (95% CI) | P Value | Odds Ratio (95% CI) | P Value | Odds Ratio (95% CI) | P Value | Odds Ratio (95% CI) | P Value |
| UES contractile reflex | Air | 1.2 (0.9–1.4)* | 0.06 | 3.5 (1.7–6.9)* | 0.0004 | 1.9 (1.1–3.3) | 0.02 | 1.6 (0.98–2.7) | 0.06 |
| Water | 10.9 (0.4–308) | 0.2 | 2.8 (0.6–12.3) | 0.2 | 2.2 (1.2–3.8) | 0.006 | 4.9 (1.6–15.3) | 0.006 | |
| Apple Juice | 2.6 (1.6–4.3) | 0.0001 | 0.7 (0.1–5.1) | 0.7 | 3.7 (1.7–8.4) | 0.001 | 3.1 (1.7–5.6) | 0.0002 | |
| LES relaxation reflex | Air | 3.6 (1.3–10.1) | 0.01 | 1.5 (0.6–3.3) | 0.4 | 1.3 (0.9–1.9) | 0.2 | 1.3 (1.0–1.7) | 0.04 |
| Water | 12.0 (0.1–1096.8) | 0.3 | 2.6 (0.6–12.2) | 0.2 | 1.3 (0.7–2.4) | 0.4 | 1.7 (1.1–2.8) | 0.02 | |
| Apple Juice | 12.9 (1.5–108.8) | 0.02 | 3.3 (0.5–22.2) | 0.2 | 1.2 (0.7–2.3) | 0.5 | 2.7 (1.3–5.3) | 0.006 | |
| Peristaltic reflex | Air | 30.2 (0.7–1386.3) | 0.08 | 1.5 (0.7–3.3) | 0.3 | 1.1 (0.8–1.6)* | 0.5 | 3.4 (1.4–8.2)* | 0.006 |
| Water | 0.6 (0.1–3.1) | 0.6 | 1.7 (0.1–22.7) | 0.7 | 1.9 (0.9–4.0) | 0.1 | 2.8 (1.6–4.9) | 0.0002 | |
| Apple Juice | 1.1 (0.5–2.5)* | 0.8 | 190.7 (5.5–6578.4)* | 0.003 | 1.7 (0.9–3.2) | 0.1 | 2.6 (1.3–5.3) | 0.006 | |
Values are expressed as odds ratio and 95% confidence intervals;
P < 0.05, maturational difference between time 1 and time 2.
Multiple logistic regression and GEE methods were applied to evaluate the relationship between the response (binary outcome of the three specific reflexes, i.e., UES contractile reflex, LES relaxation reflex, Peristaltic reflex) and the independent variables (maturational stages, sleep stages, media, and graded volume). Significant odds ratio indicate a positive correlation between volume increments and the predictive reflex. For example, with a unit increase in dose volume of air, the occurrence of UES contractile reflex with awake stage at time-1 study was 1.2 times versus 3.5 times at time-2 study, and the dose-response relationship is advanced with maturation.
Response acuities and response magnitudes of UES contractile reflex, LES relaxation reflex, and peristaltic reflex at a constant stimulus during time 1 and time 2.
Keeping the stimulus constant (1 ml), we next examined the sensory thresholds and response magnitude and compared for differences between awake vs. sleep states, for UES contractile reflex, LES relaxation reflex, and peristaltic reflex. The composite response frequency was 90%. The response onset to UES contractile reflex between the awake vs. sleep stages were measured at time 1 study (2.6 ± 1.0 vs. 4.1 ± 0.6, P = 0.5) and at time 2 study (4.6 ± 1.0 vs. 3.5 ± 0.5, P = 0.6). The UES contractile reflex durations were measured at time 1 study (3.8 ± 0.9 vs. 2.9 ± 0.5, P = 0.4) and at time 2 study (3.2 ± 1.0 vs. 3.5 ± 0.5, P = 0.9). The UES contractile reflex magnitudes (mmHg) were measured at time 1 study (33.4 ± 6.5 vs. 22.4 ± 3.5, P = 0.1) and at time 2 study (28.9 ± 6.5 vs. 24.7 ± 3.4, P = 0.3). The media effect was significant (P = 0.0008) for the response onset to UES contractile reflex but not for the duration and magnitude, in that the response latency to UES contractile reflex was significantly longer for liquids than air infusions.
The response onset to LES relaxation between the awake vs. sleep stages were measured at time 1 study (4.3 ± 0.9 vs. 3.6 ± 0.5, P = 0.5) and at time 2 study (3.2 ± 1.0 vs. 3.3 ± 0.4, P = 1.0). The LES relaxation reflex durations were measured at time 1 study (8.7 ± 1.0 vs. 3.6 ± 0.8, P = 0.6) and at time 2 study (6.3 ± 2.7 vs. 5.0 ± 0.7, P = 0.4). The LES relaxation reflex magnitude (mmHg) was measured at time 1 study (14.3 ± 3.1 vs. 21.1 ± 1.7, P = 0.3) and at time 2 study (17.9 ± 4.0 vs. 18.8 ± 1.4, P = 0.8). Similar to the UES contractile reflex, the media effect was significant (P = 0.02) for the response onset to LES relaxation reflex but not for the duration and magnitude, in that liquid stimulation had resulted in significantly longer response onset to LES relaxation reflex than air infusions.
The response latency to peristaltic reflex between the awake and sleep stages were measured at time 1 study (4.1 ± 0.7 vs. 3.9 ± 0.3, P = 0.8) and at time 2 study (4.6 ± 0.6 vs. 4.2 ± 0.3, P = 0.5). The media effect was significant (P = 0.0005), in that the response onset to peristaltic reflex was longer for liquids than air.
We further differentiated between the types of peristaltic reflexes based on proportion of EDR and SP. Comparison of the occurrence frequency of EDR and SP between the awake vs. sleep states were similar at time 1 but were significantly different at time 2. Maturational difference (time 1 vs. time 2, P < 0.001) during sleep were noted in the distribution of EDR and SP, but these differences were not observed in the awake state (Fig. 4).
Fig. 4.
Recruitment (%) of deglutition and secondary peristaltic reflex responses upon midesophageal provocation during awake and sleep states and also at time 1 and time 2 maturational phases. At time 2, note the greater proportion (%) of secondary peristalsis in sleep state compared with awake state. Furthermore, during sleep, the proportion of secondary peristalsis at time 2 is also greater than that at time 1.
DISCUSSION
Sleep is a vital physiological state for normal human brain development, resting state neuronal synapses and connectivity, conservation of energy, and optimization of growth, as well as recuperation and maintenance of organ functions. However, sleep state in high-risk infants is the most susceptible period to potential internal and external threats. Pertinent to internal threats, the neonatal aerodigestive tract has unique vulnerabilities during sleep due to the potential concerns of respiratory dysregulation, GER, and either anterograde or retrograde aspiration. In this study, we focused on the interactions between esophageal mechanosensitive and chemosensitive provocations and aerodigestive reflex responses during sleep and wakeful states in premature infants. Specifically, we proved the hypothesis that the sensory-motor characteristics of aerodigestive reflexes are dependent on 1) stimulus type and volumes, 2) sleep or awake states, and 3) maturation. The UES exhibited characteristics unique to the LES based on maturation, sleep vs. awake state, and type and volume of stimulus. With advanced maturation, we noted that the stimulus resulted in increased recruitment frequency of the UES contractile response during both sleep and awake states. Additionally, during sleep, the probability of increased recruitment of the UES contractile reflex with incremental increases in infusion volumes of air, water, and apple juice was significant at both 33 wk and 37 wk PMA, suggestive of the capability of the neonate to protect the proximal aerodigestive tract with increasing volumes. This is the first study attempting to clarify the physiology of aerodigestive adaptations upon esophageal provocations during sleep in healthy thriving premature infants performed serially at two different maturational stages, thereby providing a basis for further study of neonatal aerodigestive pathologies that modify sleep or that are modifiable by sleep.
Aerodigestive sensitivity and electro-cortical arousals: effect of maturation on infant ability to maintain sleep state.
A key observation to note was a twofold increase in the frequency of electro-cortical arousals at earlier maturational age (33 wk PMA) compared with later age (37 wk PMA). About 10.5% of stimuli given during sleep at a younger age resulted in arousals, contrasting with 5.2% of stimuli at later age. Data also suggest that immature infants have heightened sensitivity, leading to activation of electro-cortical arousals interrupting sleep. Upon maturation, afferent sensitivity and response processing abilities are modulated by favoring inhibition of ascending supranuclear pathways, resulting in fewer electro-cortical responses. These observations translate clinically as well, in that, with advanced brain growth and maturation (time 2), the study infants exhibited advanced safe oromotor functional skills, further supporting the presence of neuromotor differentiation and discrimination effects, particularly during sleep. Therefore, in healthy feeding and thriving infants, we can expect less frequent arousals with fewer sleep interruptions and better handling of the provoking stimulus. An alternative explanation for the occurrence of less frequent arousals with continued growth and maturation is that, as somatic growth increases, esophageal length also increases (12), thus esophageal stimulus spread may not reach the proximal aerodigestive tract to activate a wider afferent field of excitation. In addition, there are other hierarchical esophageal and UES defensive reflexes, discussed in detail below, that also participate in thwarting the stimulus away from the direction of proximal aerodigestive tract (11). As illustrated above, with growth and development, premature infants are better able to handle provoking stimuli and avoid sleep disturbances as they age. This ability to remain asleep during an event that would have previously caused arousal has many causes, including decreased esophageal afferent sensitivity, differential neuronal processing and interactions, and also somatic changes in esophageal length preventing proximal spread of the stimulus.
Maturation advances recruitment frequency of aerodigestive reflexes: comparing sleep vs. awake states.
With advanced maturation during awake state, we noted that the stimulus resulted in an increase in recruitment frequency of only the UES contractile reflex (Table 3). During sleep state, however, increased recruitment frequencies of UES contractile reflex, LES relaxation reflex, and peristaltic reflex were evident at time 2, demonstrating greater aerodigestive protection during sleep. These findings suggest that maturation advances the regulation of UES, esophageal body peristalsis, and LES functions during sleep. Brain stem maturation is the most likely contributing factor to the regulation of observed sensory-motor interaction patterns, with similar observations reported through maturation of the central pattern generator and the respiratory rhythm regulation apparatus (4, 5, 22, 26, 35). We speculate that appropriate regulation of esophageal peristalsis and sphincteric functions protect the proximal aerodigestive tract against retrograde threats such as GER events during sleep. Our observations regarding recruitment frequency of the UES contractile reflex, LES relaxation reflex, and peristaltic reflex support the hierarchical nature of locally evoked esophageal reflexes that play a vital role in preserving sleep and protecting the aerodigestive tract by facilitating anterograde downstream clearance and preventing retrograde upstream spread of the stimulus into the aerodigestive tract.
In general, regardless of age spectrum, awake state is associated with multiple sensory inputs for processing that result in variable somatic responses (body movements), emotional responses (facial movements), and neurological responses (changes in tone). In contrast, during sleep, the infant is likely processing stimuli differently with fewer sensory inputs and fewer somatic motor responses. In the current study involving premature infants, during both awake and sleep state, we noted that the recruitment frequencies of composite esophageal reflex response (∼80%) at time 1 and 2 were similar (Fig. 3). These findings implicate that the younger premature infant has the capability to process esophageal stimulus similarly to that of the older premature infant, although the frequency of specific defensive reflexes can be different. Explicitly, in the awake state, significant maturational differences on the recruitment frequency of UES contractile reflex (P < 0.05, Table 3) were noted with air infusions (increase in recruitment frequency at time 2) and liquid provocations (decrease in recruitment frequency at time 2). This may be due to differences in physical properties in the media modifying UES functions (i.e., UES contractile reflex prevents stimulus ascent) or facilitating stimulus clearance (UES relaxation during swallowing). Similar observations were not seen with either the LES relaxation reflex or the peristaltic reflex during awake state. This is supportive of differential processing of afferent and efferent neural signals pertaining to UES and proximal esophagus skeletal muscle functions (3, 24) vs. lower esophageal body and LES smooth muscle processing (28, 29). Our results indicate that, when the infant is awake, upon provocation with air across maturation, there is an increase in the aerodigestive protective mechanism involving the UES contractile reflex. Additionally, and in further support of previously mentioned results involving arousal frequency upon maturation, during sleep, increases in UES contractile reflex, LES relaxation reflex, and peristaltic reflex frequencies were noted. Not only are these observations indicative of differential processing, but they are also implicated when answering why older infants experience fewer arousals.
Sensory discrimination ability in sleep: effect of maturation.
Aforementioned results have established that, with maturation, significant differences are evident involving neural pathways during sleep state compared with awake state. The recruitment frequency of the three reflexes of interest (UES contractile reflex, LES relaxation reflex, and peristaltic reflex) were different in sleep state between time 1 and time 2 (P < 0.05, Table 3), thereby implicating that afferent transmission related to esophageal mechanosensitivity and chemosensitivity induced motor responses is distinct at the studied ages during sleep state. Complexity can be added to this distinction when considering that infants responded differently with varying infusion type and incremental increases in infusion volume with maturation. Although the threshold volumes are similar regardless of awake or sleep state, the frequency of response is progressively greater in sleep state with either type of media (air or liquid). Thus, discriminatory ability (graded volumes) is increasing during sleep with progressive maturation (Table 3 and Table 4).
Specifically, graded stimulus volume-response relationships were evident at both time 1 and time 2 for both awake and sleep states. SP was the most frequent peristaltic response during sleep with advanced maturation (Table 4 and Fig. 4). Responses to graded increases in volume of stimuli revealed variability during awake state, whereas less variability was observed during sleep upon maturation from time 1 to time 2. This finding leads us to hypothesize that the discriminatory ability of the neonate to modify the responses is based on physico-chemical characteristics of the stimulus. This hypothesis is further supported in that the physico-chemical properties of the media had differing response acuity and response magnitudes for the studied reflexes (Table 3). Furthermore, during sleep, the probability of increased recruitment of UES contractile reflex with incremental increases in infusion volume of air, water, and apple juice was significant at both 33 wk and 37 wk PMA, suggesting the capability of the neonate to protect the proximal aerodigestive tract against aspiration with increasing volumes. Significant increases in the recruitment of the LES relaxation reflex and peristaltic reflex during sleep were noted only at 37 wk PMA. This finding suggests the notion that, during sleep, esophageal smooth muscle-mediated peristaltic contractile reflexes and LES relaxation reflex are advanced with increased maturational age. A possible explanation for these findings pertains to contractility and resting tone as mediated by cholinergic excitatory mechanisms controlling UES responses, whereas LES relaxation is mediated via inhibitory effects from vagal nerve output by means of nitric oxide or vasoactive intestinal peptide as inhibitory agent (7–9, 13, 14, 27, 33, 34). In the absence of any extraneous somatic stimulation, arousal or awake state LES relaxation reflex is less frequent at younger PMA (33 wk), and recruitment of inhibitory vagal outputs is increased with growth and maturation (37 wk). Therefore, sleep may be an important modulating factor favoring the growth and function of inhibitory pathways, facilitating LES relaxation reflex during this time period. Sleep may also be an important factor facilitating regulation of coordination of peristaltic reflexes during these ages, as modulation of this reflex exhibits similar patterns to the LES relaxation reflex. Furthermore, UES contractile reflex and occurrence of SP with advanced maturation also point toward modulation during both sleep and awake state of cholinergic output to the UES and improved regulation of regional peristaltic reflexes, such as SP.
At a given constant midesophageal stimulus (1 ml), the response latency to all reflexes was significantly longer for liquids than air infusions, symptomatic of a physico-chemical response correlation. Although the stimulus volume was identical, the average speed of infusion flow of air was eightfold faster than liquids. Moreover, liquids may cause slower distention locally compared with rapid flow of air due to the different viscosity of these two media. Interestingly, there were greater proportions of SP (distinguishing from EDR) between sleep states with advanced maturation, with greater proportions of SP in sleep (compared to awake) noted at time 2. These findings again implicate development of appropriate local sensory-motor interactions and adaptive regulatory abilities during sleep, favoring increased occurrence of SP in healthy thriving premature infants. The occurrence of EDR can coactivate multiple neural pathways modifying respiratory rhythm and deglutition apnea during swallowing (20, 22). Such regulation appears present in premature infants during sleep. Thus wider afferent activation can be a reason for arousals, as was noted with increased frequency in younger immature infants. However, the frequency of such arousals was lesser in the same infants at later maturational age in response to similar mid esophageal stimuli. Collectively, these findings support development of stimulus discrimination, leading to decreased sleep disturbance.
Important similarities and clinical relevance.
There were no differences in the threshold sensitivity for each individual reflex examined in this study at time 1 or time 2, suggesting that afferent esophageal mechano-, osmo-, and chemosensitivity is similar in both awake and sleep states during both studies. However, efferent response processing was different, as described in the earlier sections. Aerodigestive reflex recruitment frequency during active and quiet sleep stages was similar at time 1 and time 2; hence, we regarded all sleep stages as similar. However, there may be subtle differences within the sleep stages, as has been noted in adults (3). These differences may lie in the magnitude and recruitment of reflexes. Further studies with a larger distribution of infants are needed to ascertain physiological significance of various individual sleep stages in healthy infants compared with those infants who have undergone an ALTE. The variability in dose-response relationships related to aerodigestive reflexes during the awake state may be due to variable sensory integration and motor outputs commonly seen during wakeful periods. Despite similarities in the sensory thresholds and response magnitude for the three key reflexes evoked with constant stimulus (1 ml) at both time 1 and time 2 during sleep and awake states, the media effect was significant, thus suggesting that infants' esophageal mucosal receptors were able to distinguish between liquid media and air infusions by 33 wk PMA, and this persisted at a later PMA. Further studies are needed to understand physiological significance of sensory discrimination in those infants with aerodigestive compromise.
Novelty of methods applied to investigate the proposed aims.
During natural occurrences of GER events, provocation can be of variable physico-chemical characteristics of varying volumes and varying proximal spread (18, 23), and clear sensory-motor characterization of aerodigestive reflexes is not possible. In this investigation, we used novel methodology to examine the proposed aims, which is indeed the strength of this study. We applied these methods during the evaluation of PSG characteristics of sleep concurrent with esophageal manometry. We provided targeted midesophageal provocation that was precisely defined manometrically with a distinct stimulus. To test the hypothesis of this study, this approach is needed to understand the mechanosensitivity, chemosensitivity, and osmosensitivity of the neonatal esophagus. Identical experimental protocols were performed at both time periods. Furthermore, the sensory stimulus characteristics were evenly distributed across both time periods. Specifically, the distribution of dose volumes, stimulus flow rates, and proportion of stimulus in awake vs. sleep states were similar. The amount of sleep was greater than awake period at both gestational ages by natural occurrence. Data analyses were performed in a blinded fashion; sleep scoring was performed without the knowledge of the esophageal stimulus, and the esophageal motility scoring was performed without the knowledge of sleep/awake state. These methods will be of importance to clarify aerodigestive reflexes in infants with life-threatening events related to provocation of aerodigestive tract.
Conclusions.
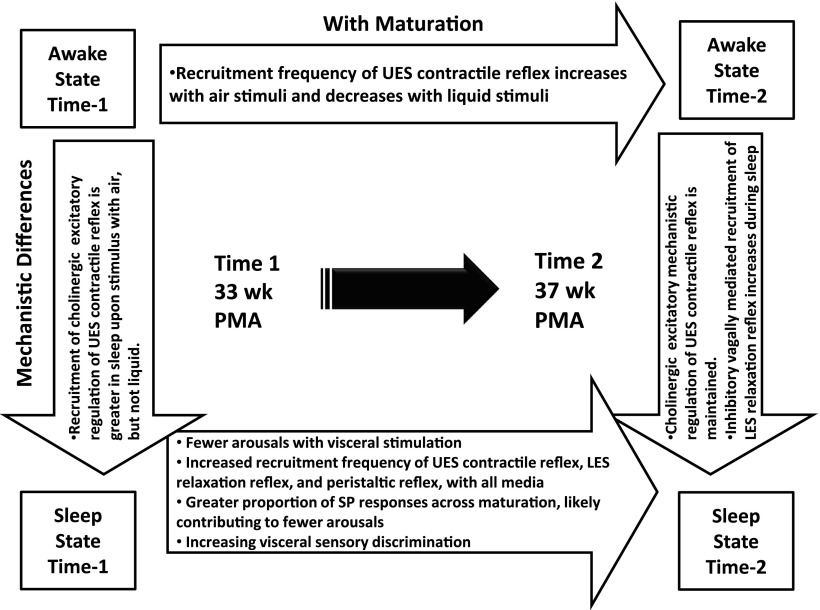
We tested the hypothesis that the sensory-motor characteristics of aerodigestive reflexes are dependent on stimulus type and volumes, sleep/awake state, and gestational age. The aerodigestive reflexes investigated were UES contractile reflex, esophageal peristaltic reflexes, and LES relaxation reflex using graded stimulus volumes (air and liquids) in different states determined during concurrent multimodal provocative esophageal manometry and video PSG in 32- and 37-wk PMA otherwise healthy infants. Sensory-motor effects of esophageal mechanosensitivity, osmosensitivity, and chemosensitivity are advanced in sleep with increased maturation. Sleep further modulates the recruitment frequency and type of aerodigestive reflexes (Fig. 5). Aerodigestive provocation occurs normally during both regular swallowing and during GER events, so airway protection and luminal clearance mechanisms are vital for survival and aerodigestive health regardless of developmental stage, i.e., immature vs. mature, or activity state, i.e., sleep vs. wakeful state. The management strategies and monitoring approaches can be different depending on the mechanisms of normality or abnormality. The premature neonates in this study were healthy and thriving. Concerns for dysphagia, aspiration, ALTE, GERD, or chronic lung disease were absent at either study times; therefore, the data from these subjects are representative of normality of adequate aerodigestive reflexes in premature infants. The presence of the tested reflexes in healthy infants provides a framework to investigate infants with aerodigestive pathologies, where these adaptive reflex responses may be abnormal.
Fig. 5.
Description of maturational and mechanistic differences during both sleep and awake state. Note that the mechanistic differences in awake vs. sleep state regarding UES contractile reflex recruitment during time 1 are maintained during time 2. Additionally, there are increased recruitment frequencies of UES contractile reflex, LES relaxation reflex, and both esophago-deglutition responses and secondary peristalsis (SP) peristaltic reflexes during sleep across maturation. PMA, postmenstrual age.
GRANTS
This study is supported in part by NIH grant RO1 DK 068158 (S. Jadcherla).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: S.R.J. and M.S. conception and design of research; S.R.J., C.Y.C., and M.S. performed experiments; S.R.J., C.Y.C., and S.F. analyzed data; S.R.J., C.Y.C., and S.F. interpreted results of experiments; S.R.J. and C.Y.C. prepared figures; S.R.J. and C.Y.C. drafted manuscript; S.R.J., C.Y.C., S.F., and M.S. edited and revised manuscript; S.R.J., C.Y.C., S.F., and M.S. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors are grateful to Juan Peng for statistical analysis, Sam Dzodzomenyo for polysomnography data verification, Rebecca Moore for nursing observations, Vanessa Parks for research assistance, Iris Keith for technical and polysomnography observations, and Reza Shaker for helpful advice with this study.
REFERENCES
- 1.American Academy of Pediatrics Committee on Fetus and Newborn Apnea, sudden infant death syndrome, and home monitoring. Pediatrics 111: 914–917, 2003 [PubMed] [Google Scholar]
- 2.American Academy of Pediatrics Committee on Fetus and Newborn Hospital discharge of the high-risk neonate. Pediatrics 122: 1119–1126, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Bajaj JS, Bajaj S, Dua KS, Jaradeh S, Rittmann T, Hofmann C, Shaker R. Influence of sleep stages on esophago-upper esophageal sphincter contractile reflex and secondary esophageal peristalsis. Gastroenterology 130: 17–25, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Barlow SM. Central pattern generation involved in oral and respiratory control for feeding in the term infant. Curr Opin Otolaryngol Head Neck Surg 17: 187–193, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barlow SM, Estep M. Central pattern generation and the motor infrastructure for suck, respiration, and speech. J Commun Disord 39: 366–380, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Clark RH, Bloom BT, Spitzer AR, Gerstmann DR. Reported medication use in the neonatal intensive care unit: data from a large national data set. Pediatrics 117: 1979–1987, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Goyal RK, Chaudhury A. Physiology of normal esophageal motility. J Clin Gastroenterol 42: 610–619, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goyal RK, Martin SB, Shapiro J, Spechler SJ. The role of cricopharyngeus muscle in pharyngoesophageal disorders. Dysphagia 8: 252–258, 1993 [DOI] [PubMed] [Google Scholar]
- 9.Goyal RK, Padmanabhan R, Sang Q. Neural circuits in swallowing and abdominal vagal afferent-mediated lower esophageal sphincter relaxation. Am J Med 111, Suppl 8A: 95S–105S, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Grigg-Damberger M, Gozal D, Marcus CL, Quan SF, Rosen CL, Chervin RD, Wise M, Picchietti DL, Sheldon SH, Iber C. The visual scoring of sleep and arousal in infants and children. J Clin Sleep Med 3: 201–240, 2007 [PubMed] [Google Scholar]
- 11.Gupta A, Gulati P, Kim W, Fernandez S, Shaker R, Jadcherla SR. Effect of postnatal maturation on the mechanisms of esophageal propulsion in preterm human neonates: primary and secondary peristalsis. Am J Gastroenterol 104: 411–419, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta A, Jadcherla SR. The relationship between somatic growth and in vivo esophageal segmental and sphincteric growth in human neonates. J Pediatr Gastroenterol Nutr 43: 35–41, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harnett KM, Cao W, Biancani P. Signal-transduction pathways that regulate smooth muscle function I. Signal transduction in phasic (esophageal) and tonic (gastroesophageal sphincter) smooth muscles. Am J Physiol Gastrointest Liver Physiol 288: G407–G416, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Harnett KM, Cao W, Kim N, Sohn UD, Rich H, Behar J, Biancani P. Signal transduction in esophageal and LES circular muscle contraction. Yale J Biol Med 72: 153–168, 1999 [PMC free article] [PubMed] [Google Scholar]
- 15.Jadcherla SR, Duong HQ, Hoffmann RG, Shaker R. Esophageal body and upper esophageal sphincter motor responses to esophageal provocation during maturation in preterm newborns. J Pediatr 143: 31–38, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Jadcherla SR, Duong HQ, Hofmann C, Hoffmann R, Shaker R. Characteristics of upper oesophageal sphincter and oesophageal body during maturation in healthy human neonates compared with adults. Neurogastroenterol Motil 17: 663–670, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Jadcherla SR, Gupta A, Coley BD, Fernandez S, Shaker R. Esophago-glottal closure reflex in human infants: a novel reflex elicited with concurrent manometry and ultrasonography. Am J Gastroenterol 102: 2286–2293, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jadcherla SR, Gupta A, Fernandez S, Nelin LD, Castile R, Gest AL, Welty S. Spatiotemporal characteristics of acid refluxate and relationship to symptoms in premature and term infants with chronic lung disease. Am J Gastroenterol 103: 720–728, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Jadcherla SR, Gupta A, Stoner E, Fernandez S, Shaker R. Pharyngeal swallowing: defining pharyngeal and upper esophageal sphincter relationships in human neonates. J Pediatr 151: 597–603, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jadcherla SR, Gupta A, Wang M, Coley BD, Fernandez S, Shaker R. Definition and implications of novel pharyngo-glottal reflex in human infants using concurrent manometry ultrasonography. Am J Gastroenterol 104: 2572–2582, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jadcherla SR, Hoffmann RG, Shaker R. Effect of maturation of the magnitude of mechanosensitive and chemosensitive reflexes in the premature human esophagus. J Pediatr 149: 77–82, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jadcherla SR, Parks VN, Peng J, Dzodzomenyo S, Fernandez S, Shaker R, Splaingard M. Esophageal sensation in premature human neonates: temporal relationships and implications of aerodigestive reflexes and electrocortical arousals. Am J Physiol Gastrointest Liver Physiol 302: G134–G144, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jadcherla SR, Peng J, Chan CY, Moore R, Wei L, Fernandez S, CDIL Significance of gastroesophageal refluxate in relation to physical, chemical, and spatiotemporal characteristics in symptomatic intensive care unit neonates. Pediatr Res 70: 192–198, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuribayashi S, Massey BT, Hafeezullah M, Perera L, Hussaini SQ, Tatro L, Darling RJ, Franco R, Shaker R. Upper esophageal sphincter and gastroesophageal junction pressure changes act to prevent gastroesophageal and esophagopharyngeal reflux during apneic episodes in patients with obstructive sleep apnea. Chest 137: 769–776, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Malcolm WF, Gantz M, Martin RJ, Goldstein RF, Goldberg RN, Cotten CM. Use of medications for gastroesophageal reflux at discharge among extremely low birth weight infants. Pediatrics 121: 22–27, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Martin RJ, Wilson CG. Apnea of prematurity. Compr Physiol 2: 2923–2931, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Mittal RK, Balaban DH. The esophagogastric junction. N Engl J Med 336: 924–932, 1997 [DOI] [PubMed] [Google Scholar]
- 28.Mittal RK, Padda B, Bhalla V, Bhargava V, Liu J. Synchrony between circular and longitudinal muscle contractions during peristalsis in normal subjects. Am J Physiol Gastrointest Liver Physiol 290: G431–G438, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Mittal RK, Rochester DF, McCallum RW. Electrical and mechanical activity in the human lower esophageal sphincter during diaphragmatic contraction. J Clin Invest 81: 1182–1189, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pena EM, Parks VN, Peng J, Fernandez SA, Di Lorenzo C, Shaker R, Jadcherla SR. Lower esophageal sphincter relaxation reflex kinetics: effects of peristaltic reflexes and maturation in human premature neonates. Am J Physiol Gastrointest Liver Physiol 299: G1386–G1395, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scher MS. Ontogeny of EEG-sleep from neonatal through infancy periods. Sleep Med 9: 615–636, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Scher MS, Steppe DA, Dahl RE, Asthana S, Guthrie RD. Comparison of EEG sleep measures in healthy full-term and preterm infants at matched conceptional ages. Sleep 15: 442–448, 1992 [DOI] [PubMed] [Google Scholar]
- 33.Sengupta JN. Electrophysiological recording from neurons controlling sensory and motor functions of the esophagus. Am J Med 111, Suppl 8A: 169S–173S, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Sengupta JN, Petersen J, Peles S, Shaker R. Response properties of antral mechanosensitive afferent fibers and effects of ionotropic glutamate receptor antagonists. Neuroscience 125: 711–723, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Thach BT. Graded arousal responses in infants: advantages and disadvantages of a low threshold for arousal. Sleep Med 3, Suppl 2: S37–S40, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Vandenplas Y, Rudolph CD, Di Lorenzo C, Hassall E, Liptak G, Mazur L, Sondheimer J, Staiano A, Thomson M, Veereman-Wauters G, Wenzl TG, North American Society for Pediatric Gastroenterology Hepatology and Nutrition, European Society for Pediatric Gastroenterology Hepatology and Nutrition Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN). J Pediatr Gastroenterol Nutr 49: 498–547, 2009 [DOI] [PubMed] [Google Scholar]