Abstract
Temperature dysregulation is an infrequent but previously documented adverse effect of antipsychotic medications. The majority of documented cases involve durations of hypothermia of less than 24 h. We present the case of a patient on therapeutic olanzapine for bipolar disease with dehydration from gastroenteritis leading to acute kidney injury in the setting of stage III chronic kidney disease, who presented with severe hypothermia of 31.2°C (88.2°F). He required active rewarming in the intensive care unit for a total of 9 days. This is the second case report of prolonged hypothermia from olanzapine in the setting of kidney disease. Clinicians should be aware that patients with renal dysfunction may be at increased risk for prolonged hypothermia from olanzapine.
Keywords: acute kidney injury, antipsychotics, chronic kidney disease, hypothermia, medication effects, olanzapine
Introduction
Temperature dysregulation is an infrequent but previously documented adverse effect of antipsychotic medications. Although hyperthermia is more commonly linked to antipsychotics, profound hypothermia may also occur. The majority of documented cases involve short durations of hypothermia, often less than 24 h. We present the second case of prolonged hypothermia from olanzapine in a patient with renal failure published in the English literature.
Case description
An 80-year-old man with a history of insulin-dependent diabetes, stage III chronic kidney disease (CKD), and bipolar disease presented to the Emergency Department (ED) with 2 days of progressively altered mental status. The patient was too confused to provide any history, but his wife reported that he recently had gastroenteritis with resultant dehydration. He had no history of trauma and no recent changes in medication within the last 3 months. She reported that he had been living with her in the family home, maintained at normal ambient temperatures, with no environmental exposures. His medications included olanzapine 5 mg twice a day, aspirin, insulin, amlodipine, and donepezil. In the ED, his rectal temperature was 31.2°C (88.2°F), heart rate 30 beats/min, blood pressure 60/palp mmHg, respiratory rate 18 breaths/min, and oxygen saturation 99% on 15 l/min supplemental oxygen. He was alert but disoriented, diaphoretic, and in mild respiratory distress. He had dry mucous membranes and a flat jugular venous pressure. The remainder of the physical examination was within normal limits.
His potassium was 5.4 meq/l, blood urea nitrogen 11.8 mmol/l, and creatinine 150.2 µmol/l, with a creatinine clearance (CrCl) of 39 ml/min, unchanged from his baseline. His thyroid stimulating hormone, free T4, random cortisol levels, and lactate were normal. Toxicology screens were negative. His electrocardiogram was only notable for marked sinus bradycardia. His chest X-ray was normal, and his head computed tomography demonstrated no acute intracranial process.
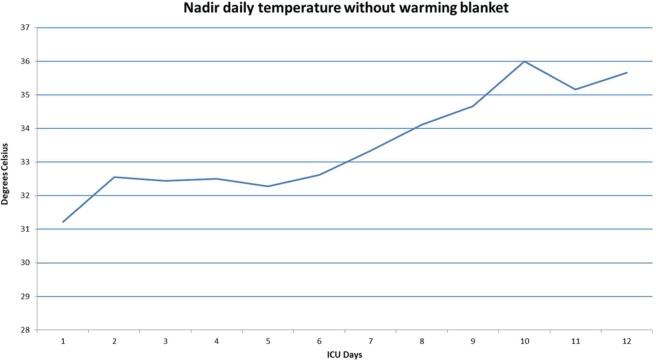
He was quickly resuscitated with warmed saline and wrapped in warm blankets. His blood pressure improved to 149/68 and his temperature improved to 32.6°C (90.8°F). He was admitted to the intensive care unit (ICU) where treatment of his hypothermia continued with warmed blankets, a forced-air warming system, and ongoing resuscitation with warmed intravenous fluids. To monitor his hypothermia, a temperature off the forced-air warming system was checked daily, and the nadir temperatures are shown in Figure 1. The timing of the removal and restoration of the warming system was left to the discretion of the bedside nurse each day. As he had no history of cold or environmental exposures, the differential diagnosis for his hypothermia included sepsis, endocrine etiologies such as myxedema coma or adrenal crisis, central nervous system pathologies, and medication effect.
Figure 1.
Daily nadir temperatures off warming blanket, in degrees Celsius.
To evaluate the etiology of his hypothermia and altered mental status, he was empirically treated for sepsis of unknown source with broad-spectrum antibiotics after blood, urine, and sputum cultures were sent. A lumbar puncture, an electroencephalogram, and brain magnetic resonance imaging were performed to rule out a neurologic cause, with all results being unremarkable. Given his unrevealing evaluation, all nonessential medications, including olanzapine, were held. Antibiotics were discontinued after 6 days when no source of infection was found and all cultures were without growth.
Over the next 2 days after admission, his CrCl dropped to 16 ml/min, despite adequate resuscitation. He was anuric for the first 4 h after arrival in the ICU. His urine output initially responded well to intravenous fluid hydration, but dropped to less than 500 ml over 24 h on ICU day 4. Nephrology was consulted and felt that his acute kidney injury (AKI) was most consistent with acute tubular necrosis (ATN) complicating CKD. With conservative management, his oliguric AKI resolved slowly with improved urine output by ICU day 6, and he did not require dialysis.
The hypothermia began to improve on day 7, with a mean daily temperature of 35°C off the warming blanket, but with continued falls in temperature to 33.3°C. His temperature normalized without intermittent episodes of hypothermia on hospital day 9 with a mean temperature of 36.1°C (97°F). With improvement in his temperature, the patient’s heart rate increased to the 60s. His CrCl never recovered, remaining in the 15–20 ml/min range. Olanzapine and all other atypical antipsychotics were permanently discontinued. He was eventually discharged to a rehabilitation facility after a 15-day hospitalization. As other causes of hypothermia including environmental exposure, myxedema coma, neurologic malignancy, adrenal insufficiency, and sepsis had been excluded, olanzapine use in the setting of CKD complicated by AKI was concluded to be the cause of his prolonged hypothermia.
Discussion
Thermoregulation occurs in the preoptic region of the anterior hypothalamus through multiple mechanisms [van Marum et al. 2007; Kreuzer et al. 2012b]. Olanzapine’s antagonism to dopaminergic D1, D2, D4, serotoninergic 5-HT2A, 5-HT2C, histaminergic H1, cholinergic M1–M5, and α1-adrenergic receptors results in multiple, occasionally conflicting, clinical symptoms in cases of acute poisoning [Ciszowski et al. 2011]. Although clinicians are familiar with the risks of development of hyperthermia and malignant neuroleptic syndrome with antipsychotic medications, hypothermia is also a serious and unpredictable adverse reaction [Blass and Chuen, 2004; Ciszowski et al. 2011]. Hypothermia due to antipsychotics may be severe, resulting in hospitalization and possibly death [Kreuzer et al. 2012b]. A review of 480 cases of hypothermia associated with the use of antipsychotic medications from the World Health Organization (WHO) database concluded that patients are at highest risk for hypothermia in the first few days after starting or after increasing the dose of antipsychotics [van Marum et al. 2007].
Patients with normal mental status will sense changes in temperature regulation and commence protective behaviors to reduce hypothermia. However, those on atypical antipsychotics may have a blunted physiologic response or exhibit apathy to initiating personal protection against the cold [van Marum et al. 2007; Kreuzer et al. 2012b]. In addition, many authors hypothesize that under normal conditions, dopaminergic and serotonin receptor activity, which decrease and increase body temperature, respectively, are balanced. Atypical antipsychotics block serotonergic receptors, thereby leading to unopposed activity of dopaminergic receptors and increasing the risk for developing hypothermia. In humans, atypical antipsychotics are linked with 55% of reported hypothermia cases associated with antipsychotic medications [van Marum et al. 2007]. Olanzapine is an atypical antipsychotic primarily designed for the treatment of schizophrenia and bipolar disease, and is the most commonly prescribed antipsychotic for bipolar disease in the USA [Baldessarini et al. 2008].
There have been 44 cases of hypothermia linked to olanzapine in WHO’s adverse drug reaction database [van Marum et al. 2007], and a review of the available literature reveals 10 prior case reports of olanzapine-induced hypothermia, including 3 cases published in a series of antipsychotic-induced hypothermia (Table 1). Hypothermia related to olanzapine has been documented in patients from the age of 0–90 years [van Marum et al. 2007: Table 2]. In the database, a diagnosis of schizophrenia is a risk factor for hypothermia and is a prevalent diagnosis among case reports [van Marum et al. 2007]. Other risk factors for hypothermia associated with antipsychotic use include medical conditions such as endocrine disease, specifically hypothyroidism, and organic brain disease including developmental delay and epilepsy [Kreuzer et al. 2012b]. Combinations of antipsychotics as well as comedication with benzodiazepines or beta blockers may increase the risk, although it is unclear if the association is due to patients with more refractory illnesses being at higher risk of disordered thermoregulation [Kreuzer et al. 2012b].
Table 1.
Comparison of published cases of olanzapine-induced hypothermia in the English literature.
| Case | Age/sex | Diagnosis | Baseline daily olanzapine dosage | Duration of stable olanzapine dosage | Dose of olanzapine prior to hypothermia | Comedications | Time to development of hypothermia after dose initiation or change | Duration of hypothermia after olanzapine | Lowest temperature in degrees Celsius | Renal function |
|---|---|---|---|---|---|---|---|---|---|---|
| Kreuzer et al. 2012b | 51 F | Catatonic schizophrenia | 30 mg | 2 weeks | 30 mg | Lorazepam | Few hours | Not reported | 30.0 | Not reported |
| Kreuzer et al. 2012b | 48 F | Schizophrenia | Haloperidol 5 mg | 6 months | 10 mg | Lorazepam | Not reported | Not reported | 31.0 | Not reported |
| Kreuzer et al. 2012b | 69 M | Acute delusional disorder | None | None | 10 mg | None | Not reported | Not reported | 33.0 | Not reported |
| Rasnayake et al. 2011 | 42 M | Schizophrenia | 10 mg | 6 years | 10 mg | Not reported | Not reported | 24 h | 32.0 | Normal, creatinine not reported |
| Hung et al. 2009 | 17 M | Schizophreniform disorder | 10 mg | 1 day | 5 mg intramuscularly × 1 | Clonazepam | 2 h | 2 h | 34.9 | Normal, creatinine not reported |
| Blass and Chuen,2004 | 64 F | Bipolar disorder | 2.5 mg | Not reported | 5 mg every morning, 6.5 mg every evening | Gabapentin | 7 days | 24 h | 33.4 | Normal, creatinine 1.2 |
| Fukunishi 20037 | 54 M | Acute delirium | 2.5 mg | 21 days | 2.5 mg | None | 2 days | 6 days | 33.0 | End-stage renal disease on hemodialysis |
| Hagg et al. 2001 | 73 M | Schizophrenia | None | None | 10 mg | None | 4 h | 36 h | 31.7 | Abnormal for system, creatinine 1.35 |
| Parris et al. 2001 | 83 F | Bipolar disorder | 5 mg | 3 weeks | 5 mg | Lithium carbonate, clonazepam and trazodone | Not reported | 24 h | 33.1 | Normal, creatinine 1.0 |
| Phan et al. 1998 | 37 F | Prader–Willi and psychosis | None | None | Not reported | Prednisone, flucloxacillin | Not reported | Not reported | 34 | Not reported |
As with other antipsychotics, most of the cases of hypothermia occur after initiation or dose increase of olanzapine, although no single unifying etiology has been identified. Of these case reports, only four patients had previously been taking olanzapine for at least a few weeks [Parris et al. 2001; Fukunishi, et al. 2003; Blass and Chuen, 2004; Rasnayake et al. 2011]. In one case, the patient had 1 day of the medication orally prior to developing hypothermia, but received an intramuscular dose prior to hypothermia [Hung et al. 2009]. In 5 out of the 10 cases, the patients received a one-time dose of olanzapine prior to developing hypothermia [Phan et al. 1998; Hägg et al. 2001; Kreuzer et al. 2012a, 2012b], including a patient who had been on haloperidol for 6 years without issues, but became hypothermic after a dose of olanzapine [Kreuzer et al. 2012b].
Olanzapine has a mean half-life of 33 h (range 21–54 h) [Collaghan et al. 1999]. It is metabolized by cytochrome P450 1A2 and has first-order elimination after multiple doses. It is highly protein bound and is excreted in urine (60%) and feces (30%), with 7% as unmetabolized drug [Collaghan et al. 1999]. Our patient had prolonged hypothermia lasting 9 days after his last dose of olanzapine, which could be explained by olanzapine’s long half-life, large volume of distribution of 1000 L, and predominantly renal excretion. Furthermore, our patient was also dehydrated secondary to a bout of gastroenteritis prior to admission. Although his initial presenting CrCl was in his baseline range, he sustained AKI with ATN, and his CrCl dropped rapidly over the next several days.
Only one previous case of a patient with renal failure developing hypothermia due to olanzapine has been reported, specifically a report from 2003 of a patient on hemodialysis [Fukunishi et al. 2003]. As with our patient, the previously reported patient was hypothermic for 6 days after his final dose of olanzapine. Notably, olanzapine is not removed by dialysis [Eli Lilly, 1996], which explains why this patient may have had a protracted course of hypothermia despite receiving renal replacement therapy. Another patient with acute kidney injury, a 73-year-old male with a CrCl greater than the upper limit of normal for the reporting institution, had the next longest duration of hypothermia at 36 h. These prolonged durations of hypothermia contrast to most other cases, in which hypothermia lasted less than 24 h.
A patient with olanzapine-induced hypothermia may be at risk for recurrent hypothermia with rechallenge. The existing case reports show that some of the patients with hypothermia due to atypical antipsychotics had previous similar reactions to other antipsychotics, including a patient with a previous hypothermic reaction to haloperidol, another with prior hypothermia after benperidol and levomepromazine [Kreuzer et al. 2012b], and a patient with three episodes of hypothermia after receiving haloperidol and levomepromazine, a single dose of 10 mg of olanzapine, and an oral dose of 2.5 mg of haloperidol, respectively [Hägg et al. 2001].
Conclusion
Patients taking antipsychotic medications, especially atypical antipsychotics, are at risk for hypothermia, a potentially life-threatening complication. Patients with renal dysfunction may be at increased risk for prolonged hypothermia from olanzapine. Clinicians should be aware of this potential medication effect, and prompt management of hypothermia before severe complications arise is critical.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declares that there is no conflict of interest.
Contributor Information
Ankit Kansagra, North Shore Medical Center, Salem, MA, USA.
Sanket Patel, Merrimack Valley Hospital, Haverhill, MA, USA.
Susan Renee Wilcox, Massachusetts General Hospital, 55 Fruit St, Boston, MA 02114, USA.
References
- Baldessarini R., Henk H., Sklar A., Chang J., Leahy L. (2008) Psychotropic medications for patients with bipolar disorder in the United States: polytherapy and adherence. Psychiatr Serv 59: 1175–1183 [DOI] [PubMed] [Google Scholar]
- Blass D., Chuen M. (2004) Olanzapine-associated hypothermia. Psychosomatics 45: 135–139 [DOI] [PubMed] [Google Scholar]
- Ciszowski K., Sein Anand J., Wilimowska J., Jawień W. (2011) The clinical picture of acute olanzapine poisonings. Przegl Lek 68: 426–433 [PubMed] [Google Scholar]
- Collaghan J., Bergstrom R., Ptak L., Beasley C. (1999) Olanzapine pharmacokinetic and pharmacodynamic profile. Clin Pharmacokinet 37: 177–193 [DOI] [PubMed] [Google Scholar]
- Lilly Eli. (1996) Zyprexa package insert. Indianapolis, IN: Eli Lilly and Co [Google Scholar]
- Fukunishi I., Sato Y., Kino K., Shirai T., Kitaoka T. (2003) Hypothermia in a hemodialysis patient treated with olanzapine monotherapy. J Clin Psychopharmacol 23: 314. [DOI] [PubMed] [Google Scholar]
- Hägg S., Mjörndal T., Lindqvist L. (2001) Repeated episodes of hypothermia in a subject treated with haloperidol, levomepromazine, olanzapine, and thioridazine. J Clin Psychopharmacol 21: 113–115 [DOI] [PubMed] [Google Scholar]
- Hung C., Huang T., Lin P. (2009) Hypothermia and rhabdomyolysis following olanzapine injection in an adolescent with schizophreniform disorder. Gen Hosp Psychiatry 31: 376–378 [DOI] [PubMed] [Google Scholar]
- Kreuzer P., Landgrebe M., Hajak G., Burger S., Langguth B. (2012a) A case of severe hypothermia following single-dose administration of olanzapine: a case report. J Clin Pharmacol 52: 266–268 [DOI] [PubMed] [Google Scholar]
- Kreuzer P., Landgrebe M., Wittmann M., Schecklmann M., Poeppl T., Hajak G., et al. (2012b) Hypothermia associated with antipsychotic drug use: a clinical case series and review of current literature. J Clin Pharmacol 52: 1090–1097 [DOI] [PubMed] [Google Scholar]
- Parris C., Mack J., Cochiolo J., Steinmann A., Tietjen J. (2001) Hypothermia in 2 patients treated with atypical antipsychotic medication. J Clin Psychiatry 62: 61–63 [DOI] [PubMed] [Google Scholar]
- Phan T., Yu R., Hersch M. (1998) Hypothermia induced by risperidone and olanzapine in a patient with Prader–Willi syndrome. Med J Aust 169: 230–231 [DOI] [PubMed] [Google Scholar]
- Rasnayake L., Wimalarathne H., Jayapala R., Gamage C., Dassanayake D., Ratnayake S., et al. (2011) An unusual case of hypothermia associated with therapeutic doses of olanzapine: a case report. J Med Case Rep 5: 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Marum R., Wegewijs M., Loonen A., Beers E. (2007) Hypothermia following antipsychotic drug use. Eur J Clin Pharmacol 63: 627–631 [DOI] [PMC free article] [PubMed] [Google Scholar]