Abstract
Neocortex is an important part of the mammalian brain that is quite different from its homologue of the dorsal cortex in the reptilian brain. Whereas dorsal cortex is small, thin, and composed of a single layer of neurons, neocortex is thick and has six layers, while being variable across species in size, number of functional areas, and architectonic differentiation. Early mammals had little neocortex, with perhaps 20 areas of poor structural differentiation. Many extant mammals continue to have small brains with little neocortex, but they often have sensory specializations reflected in the organization of sensory areas in neocortex. In primates, neocortex is variously enlarged and characterized by structural and other specializations, including those of cortical networks devoted to vision and visuomotor processing.In humans, neocortex occupies 80% of the volume of the brain, where as many as 200 areas may exist.
Keywords: primates, reptiles, dorsal cortex, visual cortex, marsupials, monotremes
Introduction
Studies of brains of various living animals indicate that several trends have probably characterized the evolution of the vertebrate nervous system. These include: (a) the development of new neural circuits; (b) progressive modification of existing ones by increase or decrease in size, number, or complexity of connections and internal organization; and (c) disappearance of existing neural circuits. Moreover, paleoneurological as well as neoneurological evidence indicates that different portions of the nervous system have exhibited different trends during the time course of evolution of a particular animal group Welker et al. (1964, Ref. 41).
Although the cerebellum has more neurons, neocortex occupies about 80% of the size of the human brain and is the critical organ of thought and consciousness.1 We differ from mice and most other mammals by having a huge brain of mostly neocortex, which is subdivided into a large number of the cortical areas that the early investigator, Brodmann, called the “organs of the brain.”2 Although some cortical areas have been well defined so that there is widespread agreement on their existence and boundaries, most are not. Thus, the areal organization of neocortex remains incompletely understood, especially in mammals with a large amount of neocortex and many cortical areas, as is the case with humans. Yet estimates place the number of cortical areas at as many as 200 or more.3,4 In contrast, early mammals had small brains with proportionately little neocortex that contained few, perhaps 20 or less, functional divisions. Neocortex is basically a sheet of tissue that typically varies only two to five times in thickness, but greatly in surface area, and when we compare the surface areas of neocortex across mammals, we see the great difference between values for one hemisphere of small, early mammals (1–1.5 cm2, see Fig. 1) and humans (800 cm2).1 Variations in the number of neurons are in the range of 2,000-fold. Thus, the neocortex of one hemisphere in the tiny smoky shrew has about 3.5 million neurons, while in humans, the neocortex has about 8 billion neurons.1,5
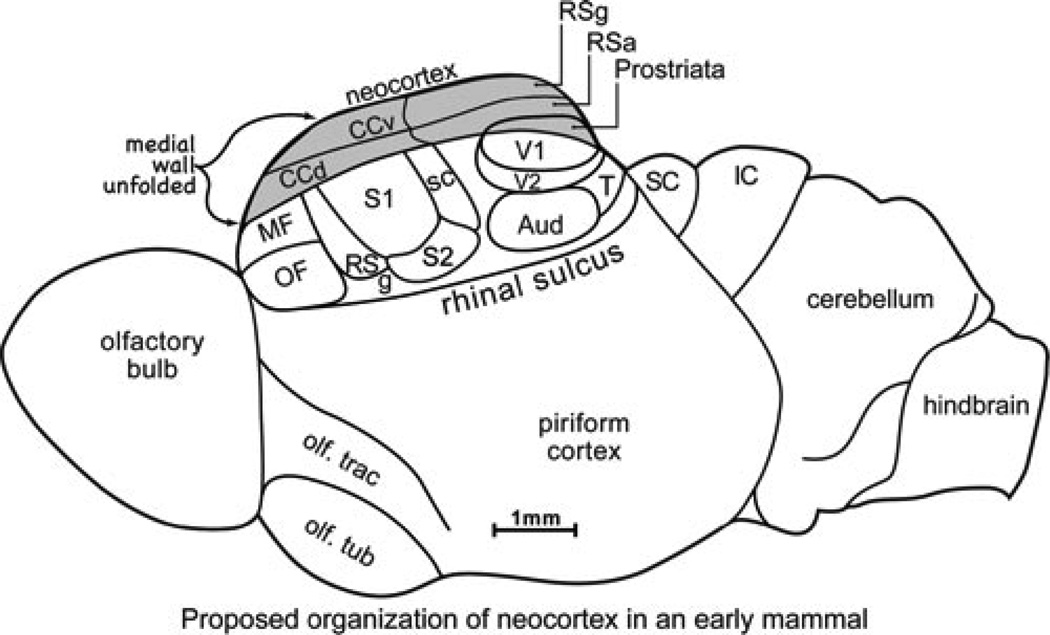
Figure 1.
The proposed organization of the neocortex of an early mammal based on a cladistic analysis of the cortex of extant mammals and proportions of neocortex reflected in the endocasts of the skulls of early mammals. Neocortex was small relative to olfactory (piriform) cortex together with the olfactory bulb. Areas of neocortex included primary somatosensory area S1 flanked by rostral (RS) and caudal (CS) somatosensory fields and a second somatosensory area, S2. A taste or gustatory area (g) may have been present. Auditory cortex (Aud) included one (A1) or two primary areas and possibly secondary areas. Visual cortex included a primary area (V1), a secondary area (V2), a temporal visual field (T), and prostriata. Frontal cortex included orbital frontal (OF) and medial frontal (MF) areas but no motor cortex. Cortex of the medial wall of the cerebral hemisphere included granular (RSg) and agranular (RSa) areas, and at least dorsal (CCd) and ventral (CCv) divisions of cingulate cortex. For reference, the superior colliculus (SC) and the inferior colliculus (IC) of the midbrain are shown
Cortical areas differ from each other in many ways, including connections with other structures, laminar and cellular structure and function, proportional representations of different parts of receptor sheets, and neuron densities.6 In a similar manner, homologous cortical areas across species may differ from each other. All of this variation raises a number of obvious questions. Thus, we wonder about the origin of the neocortex. Why did the neocortex become such an important part of the brain, especially in humans, while remaining much less so in most other mammals? What are the advantages and disadvantages of having larger brains with more neocortex? Here is the start toward addressing a few basic questions about neocortex.
Where did the neocortex come from?
Neocortex is not a new part of the brain, as it is homologous to the dorsal cortex in reptiles.6–8 However, dorsal cortex was not always recognized as a homologue of neocortex because dorsal cortex is a small region of the forebrain that consists of a thin layer of pyramidal neurons together with a few inhibitory interneurons. The pyramidal neurons of dorsal cortex receive thalamic inputs on apical dendrites that extend to the cortical surface, and have axons that project subcortically. Sensory inputs include those from the visual and somatosensory thalamus,7 with each neuron activated by many inputs. In contrast, neocortex is a layered sheet that includes, for most areas, five layers of cells and one layer of mostly axon endings and apical dendrites of cortical neurons. Neocortex receives its major activating input on small stellate neurons of layer 4, where only a few inputs provide the critical activation of each neuron. Layer 4 neurons do not provide a direct output but instead provide the main activation of neurons in a column above and below themselves. Cortical pyramidal cells project not only subcortically, but also to other areas of cortex.
The homology between dorsal cortex and neocortex was recognized in part because of the location of dorsal cortex between homologues of the hippocampus and piriform cortex, and the fact that the laminar organizations of the hippocampus and piriform cortex in mammals are more similar to their homologues in reptiles, allowing them to be more easily recognized.7
While it is tempting and perhaps reasonable to assume that the immediate, nonmammalian ancestors had a dorsal cortex much like that of present-day reptiles, this is far from certain. Early reptiles, now most often called “stem amniotes,” emerged from amphibians about 340 million years ago (mya) and soon divided into two major clades, the sauropsid clade and the synapsid clade. The sauropsid clade led to modern reptiles and birds, while the synapsid clade, now referred to as synapsids, led to early mammals perhaps 280 mya.6 We do not really know what happened to neocortex over the 40–60 million years of evolution both in the synapsid line or in early periods of sauropsid evolution before the divergence of crocodiles, turtles, snakes, lizards, and tuatara. However, similarities in dorsal cortex organization across these groups suggest that dorsal cortex had largely retained the basic features of dorsal cortex of early amniotes. Only dorsal cortex of birds, the sister clade to crocodilians, changed greatly, where it became a thicker nuclear structure7 rather than a laminated structure, as in mammals. As the synapsid line left no survivors other than mammals, we do not know from comparative studies what changes occurred to produce the laminated structure of neocortex with many more neurons. While comparative studies of development suggest ways in which the increase in neurons could have been generated,8,9 they do not fully address how the specialized layers emerged or especially how thalamic activation of the apical dendrites of pyramidal cells in a surface layer of cortex in the ancestors of mammals changed to the activation of layer 4 stellate cells in mammals. However, these changes were fundamental in the transformation of a type of dorsal cortex to a neocortex with laminar and areal specializations, vertical columnar processing, and serial and parallel processing within neocortex. The neocortex that existed in early mammals was one with great flexibility, so that many different types of cortical organization subsequently evolved.
What was the neocortex of early mammals like?
We can infer what the neocortex of early mammals was like by considering the fossil record, and by comparing cortical organization across the major branches of the mammalian radiation. Early mammals were small, mouse to cat sized, and remained so until 65 mya when conditions changed with the demise of the “ruling reptiles,” which allowed the evolution of large mammals.10 Not only were the early mammals small, but the endocasts of their skulls indicate that they had smaller brains in proportion to their body size.11 Furthermore, in favorable preservations, the locations of the rhinal fissure, a shallow groove in these fossils, can be located, and its position between neocortex and piriform cortex indicates that neocortex was especially small relative to the olfactory bulb and olfactory (piriform) cortex.12 The neocortex appeared as a small cap on the top of the forebrain, not much more extensive than the dorsal cortex of reptiles, although presumably laminated and thicker. Most extant mammals have proportionately more neocortex, although some have only slightly more.11
Comparisons across the major clades of extant mammals identify common features of neocortical organization, and it is more parsimonious to infer that these common features were retained from a common, early mammal ancestor, than to infer their independent evolution.13,14 Yet caution is needed as similar features of neocortex clearly have evolved independently.15 Early mammals diverged into six major clades leading to present-day prototherian monotremes (platypus, echidna), metatherian marsupials (opossums, kangaroos), and four groups of eutherian placental mammals, the xenarthrans (armadillos, sloths), the afrotherians (tenrecs, manatees, elephants), laurasiatherians (shrews, bats, carnivores, ungulates), and euarchontoglires (rodents, tree shrews, primates). If we focus on cortical features common to members of these six clades, and especially consider the brains of small mammals that might have changed the least since the time of the first mammals, we can reconstruct the probable features of the neocortex of the first mammals, as well as subsequent features at major branch points.13,16
The fossil record11,12 indicates that the proportion of the forebrain occupied by the neocortex in early mammals was small, while the olfactory structures, the olfactory bulb, and piriform cortex, were proportionately large (Fig. 1). As all or nearly all mammals have primary visual, auditory, and somatosensory fields,13 V1 and S1 were present. Primary cortex (Aud) included the primary area, A1, and possibly the primary-like anterior auditory field, AAF, which has been described in members of the euarchontoglire and laurasiatherian radiations, but it remains undocumented in the other radiations.17 A fringe of secondary auditory cortex of one or more fields probably bordered much of primary auditory cortex, but this has not been established by comparative studies. More can be inferred about the somatosensory cortex.14 Neurons in S1 responded well to tactile stimuli (touch). As in most extant mammals, the primary area, S1, represented the contralateral body surface from tail to tongue in a mediolateral sequence. S1 projected to narrow bands of cortex on the rostral and caudal borders of S1. The rostral band likely received proprioceptive inputs from the thalamus, and had more pronounced motor functions than S1. The caudal band was a secondary somatosensory area that distributed somatosensory information to other areas of cortex and subcortical nuclei. The second somatosensory area, just lateral to S1, received somatosensory inputs from both S1 and the ventroposterior nucleus of the somatosensory thalamus, while representing the contralateral body surface from head to foot in a dorsoventral sequence. Early mammals might have also had a parietal ventral somatosensory area, PV, which has been described in a number of mammalian species, but not in enough clades to provide clear evidence for its presence in early mammals. PV, just rostral to S2, represents the contralateral body surface as a mirror image of S2. A taste or gustatory (G) region of cortex just ventral or adjacent to S2 (Fig. 1) may be part of the basic plan, but there is little comparative evidence.
In addition to primary visual cortex, V1, there is evidence for a second visual area, V2, from a wide range of mammals18 where it is highly probable that V2 has been retained from an early mammal ancestor. V2 constitutes a second representation of the contralateral visual hemifield, reversing in retinotopy along the V1/V2 border that represents the vertical meridian or line of decussation of the retina. At least a small adjoining region of temporal cortex is visual in most studied mammals, and a visual area medial to V1 and next to retrosplenial cortex, area prostriata (Fig. 1) has been reported enough to suggest that it was present in early mammals.18
Primary motor (M1) and premotor areas were likely not present in the neocortex of early mammals, as studies in opossums have not produced evidence for any motor area rostral to somatosensory cortex,19–21 although there is mixed evidence for and against the presence of M1 in other marsupials and in monotremes.22,23 The bulk of the evidence suggests that motor cortex emerged as a separate cortical area or areas with the advent of placental (eutherian) mammals, and it did not exist in early mammals. Another trait that clearly emerged with placental mammals was the corpus callosum as a way, in addition to the anterior commissure, of connecting the neocortical areas of the two cerebral hemispheres.
Sensory and motor areas are easiest to identify as homologous across taxa, but other areas were also retained in present-day mammals from a common ancestor. They likely include agranular (dorsal) and granular (ventral) retrosplenial areas that are architectonically distinct and identified in a range of mammals.24 Frontal cortex most probably had medial frontal and lateral orbitofrontal divisions, although it has been difficult to define homologous areas of frontal cortex across mammalian taxa.25 Finally, cingulate cortex occupies the rostral half of the cortex of the medial wall of the cerebral hemispheres, and this cortex has been typically divided into three or more areas.24,26
In summary, early mammals had a small cap of neocortex on a forebrain that was dominated by a large olfactory bulb and olfactory (piriform) cortex. This small amount of neocortex was divided into roughly 15–20 functionally distinct cortical areas, including primary and secondary sensory fields, as well as retrosplenial, cingulate, and frontal areas. These areas had some architectonic differences,6,13 but functional differences largely depended on differences in inputs and outputs, rather than intrinsic structural specializations. Early mammals, including the lines that led to present-day marsupials and monotremes, lacked motor and premotor areas, as these areas evolved with eutherian (placental) mammals.
Specializations of neocortex in mammals with small brains and little neocortex
Many of the lines of evolution within the six major clades led to present-day mammals that continued to be of small body size, small brain size, and proportionately little neocortex, and yet their neocortex or some part of it had become specialized in some way. One unusual type of specialization is to reduce the number of cortical areas. This type of specialization is seen in the cortex of some species of shrews (insectivores of the Laurasiatherian clade) that appear to be smaller than their ancestors and close to, or at, the lower limit in body size for small, homeothermic mammals.27 The small brain limits the costs of the metabolically expensive tissue, but brain parts can only be reduced so far before they no longer have enough neurons to perform their normal function.28 Small shrews have solved this problem by eliminating some cortical areas so that others can be maintained at a functional size in their small brains. Thus, primary somatosensory cortex (S1) immediately adjoins primary visual cortex, and secondary somatosensory cortex (S2) immediately adjoins primary auditory cortex.29 Thus, secondary visual or auditory areas do not exist.
A related specialization is to reduce or eliminate cortical areas no longer needed after adaptations to an unusual environment. Good examples come from mammals adapted to a subterranean environment where vision is of limited use, and audition is constrained. In such mammals (over 250 species), very small eyes are common as this reduces metabolic costs and problems of dirt in the eyes and infection. The microphthalmic mole-rat (Spalax ehrenbergi) has tiny eyes completely buried under the skin.30 This mole rat appears to be blind, but has a functional retina that allows circadian and other photoperiodic rhythms. The lateral geniculate nucleus and primary visual cortex persist as very small structures that are probably too small to mediate object vision, and they appear to have been taken over by auditory inputs.31
Another type of modification of cortical organization is to distort sensory representation to provide more cortical space and neurons for parts of sensory surfaces of special adaptive value. The expanded representation of central vision in primary visual cortex of a number of mammalian taxa is a good example. Cats have an expanded representation of the central retina in V1, and monkeys have an expanded representation of the fovea.32 To a large extent, this is a reflection of great regional increases in receptor and ganglion cell densities that occur in mammals with enhanced central vision, and this would be better called receptor cell matching than cortical magnification, as receptors across the retina get approximately the same cortical space. However, in monkeys, there is also some cortical magnification in that foveal receptors get more cortical space than other receptors. In addition, neuron density is higher in parts of area 17 of monkeys that represent central vision, thereby increasing even more the number of cortical neurons devoted to each receptor.33 The best example in the auditory system is from echolocating bats where a huge proportion of primary auditory cortex can be devoted to the narrow band of high frequency tones that are used for echolocation.34,35 Nevertheless, other specializations occur such as having part of primary auditory cortex representing high frequencies used in social communication in rats and mice (the ultrasonic field).36 There are many examples for the somatosensory cortex. The primitive, ancestral condition was likely an expanded representation of the parts of the face (nose and whiskers) and teeth and tongue important in capturing and evaluating prey. Enlarged representations of parts of the face and mouth have become even more expansive in some extant mammals, such as the star-nosed mole where a specialized tactile organ, the nose, consisting of thousands of tightly packed touch domes, activates the majority of primary somatosensory cortex, S1.37
Another specialization of somatosensory cortex occurs in mole-rats,38 who use enlarged frontal teeth (incisors) to excavate tunnels, carry and manipulate food, and move their young. Mole-rats not only have proportionately more of neocortex devoted to S1, but roughly 30% of S1 represents receptors activated by touching or moving the incisors. In primates, especially anthropoid primates, the proportion of S1 devoted to the forepaw (hand), which is commonly small, is greatly enlarged.39 Wally Welker and co-workers, of course, are well known for discovering the differential enlargement of the representation of the sensitive glabrous forepaw of raccoons in S1,40 and other parts of the somatosensory system.41 Finally, the tail normally has a very small representation in S1, but in monkeys such as cebus and spider monkeys that use their tail as an important tactile surface, as much as 10% (Ref. 42) to 14% (Ref. 43) of S1 represents the tail. Other examples of phylogenetic enlargements of part of the body surface in the somatosensory system were outlined by Welker.44
A completely novel way of altering cortical sensory representations is to add a new source of activation from a newly evolved class of receptor. The unusual bill of the duck-billed platypus not only contains tactile receptors, but electroreceptors that are unique to monotremes. The arrays of touch and electroreceptors on the bill both relay to S1 cortex where the representation of the bill includes alternations of modules sensitive to touch or electric current.23 This allows the platypus to detect the electrical current produced by the muscle action of prey in murky water. Thus, somatosensory cortex has become a cortex sensitive to a new stimulus in monotremes, much like the optic tectum of pit vipers became sensitive to the body heat of prey as the tectum is activated by heat as well as vision. Other receptors have led to the evolution of separate classes of modules in S1, such as in monkeys, where modules of S1 are sensitive to either slowly adapting or rapidly adapting cutaneous receptors of the hand.45
Cortical areas also become more distinctively differentiated in structure, reflecting specialization of cell types and layers.46 In early mammals, judging from the many small-brained mammals today, cortical areas were not very distinct from each other architectonically, and layers were not very distinct.24 This condition has changed markedly in many mammals so that pronounced structural differences in layers and additional cell types evolved as components of functional change. One of the most extreme changes in the lamination of cortex occurs in primary visual cortex (V1) of tarsiers, small nocturnal primates that are so specialized as visual predators on invertebrates and small vertebrates that they eat no plants. In even Nissl preparations for cell bodies, the traditional six cortical layers of V1 (area 17) of tarsiers is clearly seen as having as many as 12 distinct sublayers.47 In addition, V1 is proportionately very large, occupying about 21% of neocortex. Neuron cell sizes and densities vary little across cortical areas in mammals that appear to have changed the least since early mammals, but major changes in cell sizes48 and packing densities have evolved across areas and layers, especially in primates.33 In addition, considerable variation exists in the distribution of classes of inhibitory interneurons in cortex across species and cortical areas,49 and some cell types such as spindle neurons are found in only a few cortical areas in a few species.
Cortical areas have also differentiated in patterns of connections with other structures. For example, both S1 and S2 are activated by projections from the ventroposterior nucleus of the somatosensory thalamus in most studied mammals, but this parallel pathway has been lost in anthropoid primates so that S2 is activated only by direct and indirect serial projections from S1.50 Other evolved differences in neocortex structure were in patterns of feedback and intrinsic connections (for review, see Ref. 46).
Of course, cortex varies greatly in size and, in some species at least, numbers of cortical areas. Changes in the overall size of cortex and in the size of cortical areas has important functional implications,28 as larger brains are largely made by adding neurons and glia rather than greatly increasing the sizes of neurons and glia.9 Thus, it becomes difficult or impossible for neurons to maintain connections with the same proportion of the increased number of other neurons in the brain. In addition, longer distances between neurons in different structures would result in longer conduction times, or compensations in axon diameter that would increase the proportion of white matter in the brain. These problems are sometimes addressed by increasing the modularity of cortex, emphasizing local processing, and devoting few axons to long connections. Thus, while early mammals had few cortical visual areas, macaque monkeys appear to have as many as 35 visual areas,51 and other systems have added cortical areas as well. Perhaps this is especially the case in human brains where a large number of cortical areas likely exist. Increasing the number of cortical areas, and the number of areas with functionally distinct modules within them increases the complexity of cortical processing so that new abilities can emerge.52–54
Primate specializations in neocortex
Most orders of mammals have a characteristic areal organization of cortex that has been modified and expanded in various ways. Here we briefly outline aspects of this cortical organization for primates. Different patterns of cortical organization could be presented for carnivores or rodents.55
All primates are characterized by an expansion of visual cortex and an increase in the number of visual areas. As in other mammals, primates have V1, V2, and prostriata, but they also have V3, DL–V4, an MT complex of motion sensitive areas (MT, MTc, MST, FST, and DM), and a number of subdivisions of visual cortex in the temporal lobe. These are areas found in all studied primates.56 Likewise, there has been an expansion of motor cortex. All primates have a primary motor area, M1, but also dorsal and ventral premotor areas, PMd and PMv, a supplementary motor area, SMA, a frontal eye field, FEF, and several cingulate motor areas.57 Auditory cortex has a primary area, A1, but also primary or primary-like rostral, R, and rostral-temporal, RT, areas which are surrounded by a set of secondary areas in the auditory belt.17 Posterior parietal cortex has a similar array of action related centers for such complex movements as reaching, grasping, and body defense.58 These centers receive visual and somatosensory information for guidance and project to motor and premotor cortex. A prefrontal granular cortex is found only in primates.25 Other characteristic features of neocortex organization in primates have been discussed by Preuss.59 Of course, neocortex is not the same in all primates, as elaborations of the basic framework are apparent in Old World monkeys and especially humans, in comparison to prosimian primates. Thus, the human neocortex has additional visual areas for face or object recognition,60 posterior parietal areas specialized for tool use,61 and auditory areas devoted to language.62 Humans have major differences in the organization of the two hemispheres (hemispheric specialization) and expanded regions of frontal cortex for social cognition.59 Perhaps more than any other mammal, the expansion, subdivision, and specializations of neocortex in humans have demonstrated the great potential of neocortex; however, this potential has been realized at the cost of an extremely long developmental time and a huge ongoing metabolic cost. Most mammals do not pay these costs, as they have smaller brains with less, or much less, neocortex, with less computational ability, which is balanced by earlier and often higher rates of reproduction, and typically reduced requirements for high grade food.
Acknowledgments
This article is dedicated to the memory of Wally Welker, who guided me while I was a postdoctoral fellow and assistant professor at the University of Wisconsin, and taught me much of the value and excitement of a comparative approach to understanding brain organization and function.
Footnotes
Conflicts of interest
The author declares no conflicts of interest.
References
- 1.Avzevedo FAC, Carvalho LRB, Grinberg LT, et al. Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J. Comp. Neurol. 2009;513:532–541. doi: 10.1002/cne.21974. [DOI] [PubMed] [Google Scholar]
- 2.Brodmann K. Vergleichende Lokalisationslehre Der Grosshirnrhinde. Barth: Leipzig; 1909. [Google Scholar]
- 3.Changizi MA, Shimojo S. Parcellation and area-area connectivity as a function of neocortex size. Brain Behav. Evol. 2005;66:88–98. doi: 10.1159/000085942. [DOI] [PubMed] [Google Scholar]
- 4.Finlay B, Brodsky P. Cortical evolution as the expression of a program for disproportionate growth and the proliferation of areas. In: Kaas JH, Krubitzer LA, editors. Evolution of Nervous Systems, Vol. 3: Mammals. Oxford: Elsevier; 2007. pp. 73–96. [Google Scholar]
- 5.Sarko DK, Catania KC, Leitch DB, et al. Cellular scaling rules of insectivore brains. Front. Neuroanat. 2009;3:1–12. doi: 10.3389/neuro.05.008.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Northcutt RG, Kaas JH. The emergence and evolution of mammalian neocortex. Trends Neurosci. 1995;18:373–379. doi: 10.1016/0166-2236(95)93932-n. [DOI] [PubMed] [Google Scholar]
- 7.Medina L. Do birds and reptiles possess homologues of mammalian visual, somatosensory and motor cortices? In: Kaas JH, Bullock TH, editors. Evolution of Nervous Systems, Vol. 2: Non-Mammalian Vertebrates. Oxford: Elsevier; 2007. pp. 163–194. [Google Scholar]
- 8.Molnár Z, Tavare A, Cheung FP. The origin of neocortex: lessons from comparative embryology. In: Kaas JH, Krubitzer LA, editors. Evolution of Nervous Systems, Vol. 3: Mammals. Oxford: Elsevier; 2007. pp. 13–26. [Google Scholar]
- 9.Rakic P. A small step for the cell, a giant leap for mankind: a hypothesis of neocortical expansion during evolution. Trends Neurosci. 1995;18:383–388. doi: 10.1016/0166-2236(95)93934-p. [DOI] [PubMed] [Google Scholar]
- 10.Smith FA, Boyer AG, Brown JH, et al. The evolution of maximum body size of terrestrial mammals. Science. 2010;330:1216–1219. doi: 10.1126/science.1194830. [DOI] [PubMed] [Google Scholar]
- 11.Jerison HJ. What fossils tell us about the evolution of the neocortex. In: Kaas JH, Krubitzer LA, editors. Evolution of Nervous Systems, Vol. 3: Mammals. Oxford: Elsevier; 2007. pp. 1–12. [Google Scholar]
- 12.Kielan-Jaworowska Z, Cifelli RL, Luo Z-X. Mammals from the Age of Dinosaurs. New York: Columbia Univ. Press; 2004. [Google Scholar]
- 13.Kaas JH. Reconstructing the organization of the forebrain of the first mammals. In: Kaas JH, Krubitzer LA, editors. Evolution of Nervous Systems, Vol 3: Mammals. Oxford: Elsevier; 2007a. pp. 27–48. [Google Scholar]
- 14.Kaas JH. The evolution of sensory and motor systems in primates. In: Kaas JH, Preuss TM, editors. Evolution of Nervous Systems, Vol. 4: Primates. Oxford: Elsevier; 2007b. pp. 35–57. [Google Scholar]
- 15.Kaas JH. Convergences in the modular and areal organization of the forebrain of mammals: implications for the reconstruction of forebrain evolution. Brain Behav. Evol. 2002;59:262–272. doi: 10.1159/000063563. [DOI] [PubMed] [Google Scholar]
- 16.Kaas JH, Preuss TM. Human brain evolution. In: Squire LR, editor. Fundamental Neuroscience. 3rd ed. San Diego: Elsevier; 2008. pp. 1027–1035. [Google Scholar]
- 17.Kaas JH. The evolution of auditory cortex; the core areas. In: Winer JW, Schreiner CE, editors. The Auditory Cortex. New York: Springer-Verlag; 2011. pp. 407–427. http://www.springerlink.com/content/978-1-4419-0073-9. [Google Scholar]
- 18.Rosa MGP, Krubitzer LA. The evolution of visual cortex: where is V2? TINS. 1999;22:242–248. doi: 10.1016/s0166-2236(99)01398-3. [DOI] [PubMed] [Google Scholar]
- 19.Nudo RJ, Masterton RB. Descending pathways to the spinal cord, III: sites of origin of the corticospinal tract. J. Comp. Neurol. 1990;296:559–583. doi: 10.1002/cne.902960405. [DOI] [PubMed] [Google Scholar]
- 20.Beck PD, Pospichal MW, Kaas JH. Topography, architecture, and connections of somatosensory cortex in opossums: evidence for five somatosensory areas. J. Comp. Neurol. 1996;366:109–133. doi: 10.1002/(SICI)1096-9861(19960226)366:1<109::AID-CNE8>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 21.Frost SB, Milliken GW, Plautz EJ, et al. Somatosensory and motor representations in cerebral cortex of a primitive mammal (Monodelphis domestica): a window into the early evolution of sensorimotor cortex. J. Comp. Neurol. 2000;421:29–51. doi: 10.1002/(sici)1096-9861(20000522)421:1<29::aid-cne3>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 22.Rowe M. Organization of the cerebral cortex in monotremes and marsupials. In: Jones EG, Peters A, editors. Cerebral Cortex: Comparative Structures and Evolution of Cerebral Cortex, Part II. New York: Plenum Press; 1990. pp. 263–334. [Google Scholar]
- 23.Krubitzer L, Manger P, Pettigrew J, et al. Organization of somatosensory cortex in monotremes: in search of the prototypical plan. J. Comp. Neurol. 1995;351:261–306. doi: 10.1002/cne.903510206. [DOI] [PubMed] [Google Scholar]
- 24.Wong P, Kaas JH. An architectonic study of the neocortex of the short-tailed opossum (Monodelphis domestica) Brain Behav. Evol. 2009;73:206–228. doi: 10.1159/000225381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Preuss TM. Do rats have prefrontal cortex? The Rose-Woolsey-Akert proszgram reconsidered. J. Cogn. Neurosci. 1995;7:1–24. doi: 10.1162/jocn.1995.7.1.1. [DOI] [PubMed] [Google Scholar]
- 26.Domesick VB. Thalamic relationships of the medial cortex in the rat. Brain Behav. Evol. 1972;6:457–483. doi: 10.1159/000123727. [DOI] [PubMed] [Google Scholar]
- 27.Schmidt-Nielsen K. Scaling: Why is Animal Size So Important? Cambridge: Cambridge University Press; 1984. [Google Scholar]
- 28.Kaas JH. Why is brain size so important: design problems and solutions as neocortex gets bigger or smaller. Brain Mind. 2000;1:7–23. [Google Scholar]
- 29.Catania KC, Lyon DC, Mock OB, et al. Cortical organization in shrews: evidence from five species. J. Comp. Neurol. 1999;410:55–72. [PubMed] [Google Scholar]
- 30.Cooper HM, Herbin M, Nevo E. Visual system of a naturally microphthalmic mammal: the blind mole rat, Spalax Ehrenbergi. J. Comp. Neurol. 1993;328:313–350. doi: 10.1002/cne.903280302. [DOI] [PubMed] [Google Scholar]
- 31.Heil P, Bronchti G, Wollberg Z, et al. Invasion of visual cortex by the auditory system in the naturally blind mole rat. Neuroreport. 1991;2:735–738. doi: 10.1097/00001756-199112000-00001. [DOI] [PubMed] [Google Scholar]
- 32.Azzopardi P, Cowey A. Preferential representation of the fovea in the primary visual cortex. Nature. 1993;361:719–721. doi: 10.1038/361719a0. [DOI] [PubMed] [Google Scholar]
- 33.Collins CE, Airey DC, Young NA, et al. Neuron densities vary across and within cortical areas in primates. Proc. Natl. Acad. Sci. USA. 2010;107:15927–15932. doi: 10.1073/pnas.1010356107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suga N. Multi-function theory for cortical processing of auditory information: implications of single-unit and lesion data for future research. J. Comp. Physiol. A. 1994;175:135–144. doi: 10.1007/BF00215109. [DOI] [PubMed] [Google Scholar]
- 35.Kaas JH, Hackett TA. The functional neuroanatomy of the auditory cortex. In: Dallos P, Oertel D, editors. The Senses: A Comprehensive Reference, Vol. 3: Audition. Oxford: Elsevier; 2008. pp. 765–780. [Google Scholar]
- 36.Stiebler I, Neulist R, Fichtel I, et al. The auditory cortex of the house mouse: left-right differences, tonotopic organization and quantitative analysis of frequency representation. J. Comp. Neurol. 1997;181:559–571. doi: 10.1007/s003590050140. [DOI] [PubMed] [Google Scholar]
- 37.Catania KC, Kaas JH. Organization of the somatosensory cortex of the star-nosed mole. J. Comp. Neurol. 1995;351:549–567. doi: 10.1002/cne.903510406. [DOI] [PubMed] [Google Scholar]
- 38.Catania KC, Remple MS. Somatosensory cortex dominated by the representation of teeth in the naked molerat brain. Proc. Natl. Acad. Sci. USA. 2002;99:5692–5697. doi: 10.1073/pnas.072097999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qi HX, Kaas JH. Myelin stains reveal an anatomical framework for the representation of the digits in somatosensory area 3b of macaque monkeys. J Comp. Neurol. 2004;477:172–187. doi: 10.1002/cne.20247. [DOI] [PubMed] [Google Scholar]
- 40.Welker WI, Seidenstein S. Somatic sensory representation in the cerebral cortex of the raccoon (Procyon lotor) J. Comp. Neurol. 1959;111:469–501. doi: 10.1002/cne.901110306. [DOI] [PubMed] [Google Scholar]
- 41.Welker WI, Johnson JI, Jr., Pubols BH. Some morphological and physiological characteristics of the somatic sensory system in raccoons. Am. Zool. 1964;4:75–94. doi: 10.1093/icb/4.1.75. [DOI] [PubMed] [Google Scholar]
- 42.Felleman DJ, Nelson RJ, Sur M, et al. Representations of the body surface in areas 3b and 1 of postcentral parietal cortex of Cebus monkeys. Brain Res. 1983;268:15–26. doi: 10.1016/0006-8993(83)90386-4. [DOI] [PubMed] [Google Scholar]
- 43.Pubols BH, Pubols LM. Somatotopic organization of spider monkey somatic sensory cerebral cortex. J. Comp. Neurol. 1971;141:63–76. doi: 10.1002/cne.901410106. [DOI] [PubMed] [Google Scholar]
- 44.Welker WI. Principles of organization of the ventrobasal complex in mammals. Brain, Behav. Evol. 1973;7:253–336. doi: 10.1159/000124417. [DOI] [PubMed] [Google Scholar]
- 45.Sur M, Wall JT, Kaas JH. Modular distribution of neurons with slowly adapting and rapidly adapting responses in area 3b of somatosensory cortex in monkeys. J. Neurophysiol. 1984;51:724–744. doi: 10.1152/jn.1984.51.4.724. [DOI] [PubMed] [Google Scholar]
- 46.Kaas JH. Cortical circuits: consistency and variability across cortical areas and species. In: von der Malsburg C, Philips WA, Singer W, editors. Dynamic Coordination in the Brain: From Neurons to Mind. 2010 Strügmann Forum Report, Vol. 5. Cambridge: MIT Press; 2010. pp. 25–34. [Google Scholar]
- 47.Collins CE, Hendrickson A, Kaas JH. Overview of the visual system of Tarsius. Anat. Rec. A Discov. Mol. Cell Evol. Biol. 2005;287:1013–1025. doi: 10.1002/ar.a.20263. [DOI] [PubMed] [Google Scholar]
- 48.Elston GN, Tweedale R, Rosa MGP. Cortical integration in the visual system of the macaque monkey: large scale morphological differences of pyramidal neurons in the occipital, parietal and temporal lobes. Proc. R Soc. Lond. Ser. B. 1999;266:1367–1374. doi: 10.1098/rspb.1999.0789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hof PR, Sherwood CC. The evolution of neuron classes in the neocortex of mammals. In: Kaas JH, Krubitzer LA, editors. Evolution of Nervous Systems. Vol. 3: Mammals. Oxford: Elsevier; 2007. pp. 113–124. [Google Scholar]
- 50.Garraghty PE, Pons TP, Kaas JH. Ablations of areas 3b (S-I proper) and 3a of somatosensory cortex in marmosets deactivate the second and parietal ventral somatosensory areas. Somatosens. Mot. Res. 1990;7:125–135. doi: 10.3109/08990229009144703. [DOI] [PubMed] [Google Scholar]
- 51.Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cereb. Cortex. 1991;1:1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- 52.Cowey A. Why are there so many visual areas? In: Schmitt FO, Warden FG, Adelman G, Dennis VG, editors. Models of Visual Cortex. New York: Wiley and Sons; 1981. pp. 54–61. [Google Scholar]
- 53.Barlow HB. Why have multiple visual areas? Vision Res. 1986;26:81–90. doi: 10.1016/0042-6989(86)90072-6. [DOI] [PubMed] [Google Scholar]
- 54.Kaas JH. The evolution of complex sensory systems in mammals. J. Exp. Biol. 1989;146:165–176. doi: 10.1242/jeb.146.1.165. [DOI] [PubMed] [Google Scholar]
- 55.Lyon DC. The evolution of visual cortex and visual systems. In: Kaas JH, Krubitzer LA, editors. Evolution of Nervous Systems. Vol. 3: Mammals. Oxford: Elsevier; 2007. pp. 267–306. [Google Scholar]
- 56.Kaas JH, Lyon DC. Visual cortex organization in primates: theories of V3 and adjoining visual areas. Prog. Brain Res. 2001;134:285–295. doi: 10.1016/s0079-6123(01)34019-0. [DOI] [PubMed] [Google Scholar]
- 57.Wu CW-H, Bichot NP, Kaas JH. Converging evidence from microstimulation, architecture, and connections for multiple motor areas in the frontal and cingulate cortex of prosimian primates. J. Comp. Neurol. 2000;423:140–177. doi: 10.1002/1096-9861(20000717)423:1<140::aid-cne12>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 58.Stepniewska I, Fang PC, Kaas JH. Mi-crostimulation reveals specialized subregions for different complex movements in posterior parietal cortex of prosimian galagos. Proc. Natl. Acad. Sci. USA. 2005;102:4878–4883. doi: 10.1073/pnas.0501048102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Preuss TM. Primate brain evolution in phylogenetic context. In: Kaas JH, Preuss TM, editors. Evolution of Nervous Systems. Vol. 4: Primates. Elsevier: Oxford; 2007. pp. 1–34. [Google Scholar]
- 60.Hoffman KL, Gauthier I. Evolution of Nervous Systems. Vol. 4: Primates. Oxford: Elsevier; 2007. Evolutionary specializations for processing faces and objects; pp. 437–445. [Google Scholar]
- 61.Frey SH. Evolution of Nervous Systems. Vol. 4: Primates. Oxford: Elsevier; 2007. Neurological specializations for manual gesture and tool use in humans; pp. 395–406. [Google Scholar]
- 62.Deacon TW. The evolution of language systems in the human brain. In: Kaas JH, Preuss TM, editors. Evolution of Nervous Systems. Vol. 4: Primates. Oxford: Elsevier; 2007. pp. 529–547. [Google Scholar]