Fig. 10.
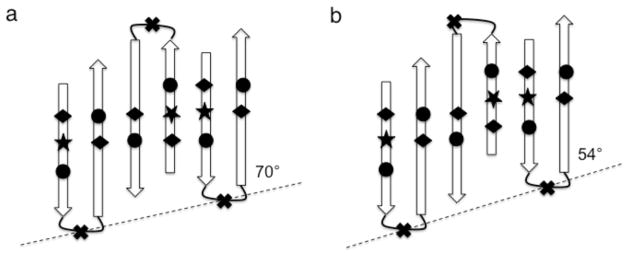
Schematic representation of two ways of forming an extended, anti-parallel β-sheet along the vesicle rib (dashed lines), employing antiparallel dimers that distribute the helix and coil segments of GvpA on both edges of the sheet. All β-turns are odd-membered so that all the non-aliphatic residues, including the unique tryptophan (★), are on the side of the sheet facing the reader and all charged residues [carboxylic acids (◆) and arginines (●)] are paired in salt bridges. (a) With a symmetric dimer unit cell in which both β-turns are centered on glycine (✖), the inclinations of the strands to the rib and the hydrogen bonds to the vesicle axis are 70°. (b) with an asymmetric dimer unit cell, in which β-turns in alternating molecules are centered on valine instead of glycine, the inclinations of the strands to the rib and the hydrogen bonds to the vesicle axis are 54°. Note that the angles do not appear to be of the labeled magnitude because the distances between strands have been rendered on a larger scale than the distances along strands in order to enhance clarity.
