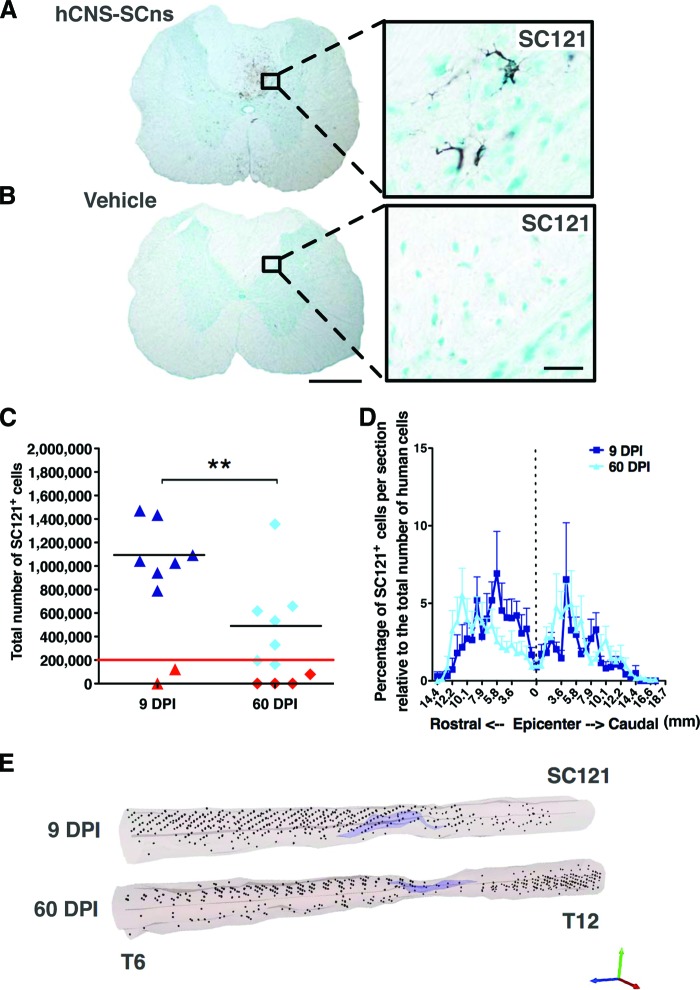
Figure 1.
hCNS-SCns engraft, survive, and migrate when transplanted at either the subacute or chronic stage of spinal cord injury (SCI). Transplanted, engrafted spinal cords (A) showed human-specific cytoplasmic marker SC121+ cells, unlike vehicle-treated cords (B) at 14 weeks post-transplantation (WPT). Scale bars = 500 μm (left) and 50 μm (right). Unbiased analysis using StereoInvestigator optical fractionator (C) revealed that the total estimated numbers of SC121+ cells were significantly lower in the 60 DPI hCNS-SCns cohort compared with those in the 9 DPI hCNS-SCns cohort (Student's two-tailed t test; **, p < .009). Red line indicates the initial transplantation dose, black line indicates the average number of SC121+ human cells, and red triangles indicate animals excluded because of poor engraftment. (D): Cell migration is shown as percentage of SC121+ cells per section relative to total number of counted human cells (Student's two-tailed t test; p > .05). Positional data are plotted relative to the injury epicenter (vertical dashed line), which was designated based on identification of the tissue section containing the largest cross-sectional lesion area. Error bars shown as SEM. (E): Three-dimensional reconstruction of spinal cords transplanted at 9 or 60 DPI. The large cavities typical for athymic nude (ATN) rat SCI model are indicated in light purple, and the locations of analyzed SC121+ human cells based on StereoInvestigator optical fractionator probe quantification data are represented by black dots. StereoInvestigator data were analyzed from coronal sections in a 1 of 24 sampling sequence at 14 WPT (StereoInvestigator data from the 9 DPI cohort in [C] were previously published in [7]). Abbreviations: DPI, days postinjury; hCNS-SCns, human central nervous system-derived neural stem cell.