Abstract
Background
HIV-infected patients are at increased risk of coronary artery disease (CAD). We evaluated the cost-effectiveness of cardiac screenings for HIV-positive men at intermediate or greater CAD risk.
Design
We developed a lifetime microsimulation model of CAD incidence and progression in HIV-infected men.
Methods
Input parameters were derived from two HIV cohort studies and the literature. We compared no CAD screening with stress testing and coronary computed tomography angiography (CCTA)-based strategies. Patients with test results indicating 3-vessel/left main CAD underwent invasive coronary angiography (ICA) and received coronary artery bypass graft surgery. In the “Stress-testing+Medication”/“CCTA+Medication” strategies, patients with 1-/2-vessel CAD results received lifetime medical treatment without further diagnostics whereas in the “Stress-testing+Intervention”/“CCTA+Intervention” strategies, patients with these results underwent ICA and received percutaneous coronary intervention.
Results
Compared to no screening, the “Stress-testing+Medication”, “Stress-testing+Intervention”, “CCTA+Medication” and “CCTA+Intervention” strategies resulted in 14, 11, 19, and 14 quality-adjusted life days per patient and incremental cost effectiveness ratios of 49,261, 57,817, 34,887 and 56,518 € per quality-adjusted life year (QALY), respectively. Screening only at higher CAD risk thresholds was more cost-effective. Repeated screening was clinically beneficial compared to one-time screening but only “Stress-testing+Medication” every five years remained cost-effective. At a willingness-to-pay threshold of 83,000 €/QALY (~100,000 US$/QALY), implementing any CAD screening was cost-effective with a probability of 75-95%.
Conclusion
Screening HIV-positive men for CAD would be clinically beneficial and comes at a cost-effectiveness ratio comparable to other accepted interventions in HIV care.
Keywords: HIV, coronary heart disease, prevention, cost-effectiveness, Markov model
Introduction
The advent of highly active antiretroviral therapy (HAART) has resulted in improved survival of human immunodeficiency virus (HIV)-infected patients.1 However, improved survival has given rise to age-related health concerns in the HIV-positive population; recent studies suggest that these patients are at increased risk for cardiac events and coronary artery disease (CAD).2-5 The Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) Study Group as well as a study by Triant et al found that HIV-infected patients are at an elevated risk of myocardial infarction (MI).3-4 Lo and colleagues found an increased prevalence of subclinical atherosclerosis in HIV-positive men.5 Currier and coworker reported an accelerated incidence of CAD among younger HIV-infected individuals.2
The increased risk for CAD in the HIV-infected population is believed to result from both a greater prevalence of traditional cardiac risk factors, e.g., greater tobacco consumption as compared to the general population, and the unfavorable effects of HIV infection, HAART, and related inflammatory and immune processes on the development of traditional CAD risk factors.6-7 As a result, professional societies such as the American Heart Association have convened experts to consider CAD screening in the HIV-infected population.8 However, there is ongoing debate as to which specific tests should be used and to what extent thresholds for initiation of screening can be directly transferred from the non-HIV-infected population, presenting the need for more clinical evidence.8
We performed a decision analysis to assess the health outcomes, costs, and cost-effectiveness of four alternative CAD screening strategies in HIV-infected men with an intermediate or greater risk of CAD compared to the standard of care (SOC), i.e., no screening.
Methods
We developed a Markov microsimulation model to evaluate CAD screening for HIV-positive men without known cardiac disease. A patient’s risk of CAD was determined by the 10-year Framingham risk score for CAD. In the base-case analysis, the screening threshold was set at a 10-year Framingham CAD risk of ≥7.5%, since, as demonstrated by the D:A:D study group, Framingham risk equations tend to underestimate cardiac risk in HIV-infected patients.3,9 We conducted sensitivity analyses on all key input variables and performed probabilistic sensitivity analysis.
Screening Strategies
In order to reflect current CAD screening recommendations, we compared no screening to CAD screening strategies that either involve functional or coronary computed tomography angiography (CCTA)-based testing. In all strategies, patients initially received an electrocardiogram (ECG) followed by either stress-testing or CCTA. The default stress-test in the general screening strategies was stress-ECG; stress-echocardiography was used if the initial ECG showed abnormalities.8
In the “Stress-testing+Medication” strategy, patients with results indicating 3-vessel (≥70% stenosis) or left main (≥50% stenosis) severe obstructive CAD (hereafter “severe CAD”) proceeded to invasive coronary angiography (ICA) and subsequently received coronary artery bypass graft (CABG) surgery and lifetime medical therapy. Patients with results indicating 1- or 2-vessel severe CAD (≥70% stenosis) received lifetime medical treatment without further diagnostics.
In the “Stress-testing+Intervention” strategy, all patients with abnormal stress-testing results proceeded to ICA. Based on the ICA findings, patients with 1- or 2-vessel severe CAD were treated with percutaneous coronary intervention (PCI); patients with 3-vessel or left main severe CAD underwent CABG surgery. Additionally, all patients diagnosed with severe CAD received lifetime medical therapy.
In the CCTA-based strategies all patients received CCTA. Depending on the disease severity as measured by CCTA, the patients proceeded in the same clinical algorithms as described above for stress-testing. A detailed depiction of the screening strategies as well as the diagnostic test characteristics and risks of cardiac interventions are provided in the appendix.
Model Pathways
After screening, patients entered a simulation model that reflected the prevalence, incidence, and progression of obstructive CAD and congestive heart failure (CHF) as well as mortality in HIV-infected men under SOC.
The 5-state Markov model distinguished between no obstructive CAD, mild/moderate obstructive CAD, severe CAD, CHF, and death (appendix). Patients could have no cardiac disease, obstructive CAD, or CHF. During annual cycles patients could develop obstructive CAD, assuming a natural progression to severe CAD via mild/moderate CAD, experience a progression in the severity of CAD, develop CHF, or die from HIV, cardiac disease, or other causes. The risks of CAD development and progression as well as CHF development depended on the patient’s age and other cardiac risk factors. Diagnosis and treatment of severe CAD was reflected in a lower risk of cardiac death in correctly identified patients. The model was built using TreeAge Pro Suite 2009 (TreeAge Software, Inc., Williamstown, Massachusetts).
Clinical Input Parameters
All patients were HIV-positive males. CAD prevalence and incidence of obstructive CAD and CHF were estimated using data on cardiac risk factors from the HIV-HEART study and data on CAD status in the HIV-infected population from a study by Lo et al.5 The HIV-HEART study assessed the cardiac status of 802 HIV-positive patients (83.4% male; age 44.3 ±10.3 years).10 We included 600 male patients (age 44.2 ±10.0 years) without known cardiac disease in our analysis. Lo and colleagues examined atherosclerosis in 78 HIV-positive men (age 46.5 ±6.5 years).5 Estimates for other input parameters were derived from the literature and German life tables.
HIV Infection
The model assumed that patients were treated according to HIV treatment guidelines.11-12 The excess mortality in the HIV-infected population compared to the non-infected population was modelled as a function of the baseline CD4 cell count. We calculated a HRR of 4.6 by weighting the mortality HRRs of 8.56, 5.24, and 3.01 reported for CD4 cell count categories of <200 cells/μl, 200-500 cells/μl, and >500 cells/μl, respectively, by the initial distribution of CD4 cells in the 600 male participants of the HIV-HEART cohort (11.8% with <200 cells/μl, 42.8% with 200-500 cells/μl, and 45.4% with >500 cells/μl).13 In our model, this HRR was applied to age- and gender-specific life table mortality rates reduced by the cardiovascular-specific mortality rates.
Coronary Artery Disease
Since the CAD status of the HIV-HEART participants was not assessed, we imputed the prevalence of obstructive CAD based on a logistic regression analysis using data collected by Lo and colleagues (appendix).5,9
The individual’s risk of developing obstructive CAD or progressing in severity was determined using the 10-year Framingham risk equation for CAD,9 assuming a natural progression from no obstructive CAD to severe CAD via mild/moderate CAD. Additionally, based on the findings of the D:A:D study group, we accounted for the increased risk of CAD due to HIV infection-related causes by applying a HRR of 1.3 to the Framingham risk;3 the patient’s CAD risk was repeatedly updated as characteristics changed over time.
The impact of treatment on the progression of severe CAD was reflected indirectly through a decrease in mortality risk which was modeled based on the number of affected vessels at diagnosis.14 Mortality HRRs for patients with treated severe CAD compared to patients without obstructive CAD were 2.23, 3.29 and 7.35 for 1-, 2- and 3-vessel/left main disease, respectively.14 The HRRs increased by a factor of 1.3 in patients with untreated severe CAD.15 For mild/moderate CAD as compared to no obstructive CAD, the mortality HRR was 1.079.16
Given that there is no evidence of a difference in excess mortality due to CAD between HIV-infected and non-infected individuals, we considered the CAD excess mortality rates to be additive to HIV-adjusted age- and gender-specific mortality rates.
Congestive Heart Failure
None of the HIV-HEART patients included in the sample were diagnosed with CHF at initial presentation. However, patients could develop CHF as a function of their Framingham risk score for CHF.17 We allowed this risk to change over time and accounted for the increased hazard of CHF due to HIV infection (as compared to the non-HIV-infected population) by applying a HRR of 1.3 to the progression rate.3
Mortality HRRs for treated CHF as compared to normal cardiac function were modeled as a function of time since CHF-onset, independent of the patient’s CAD status (appendix).18 Similar to our findings for CAD, there was no evidence that excess mortality from CHF differed between HIV-infected and non-infected populations; we added the excess mortality rates secondary to CHF to the HIV-adjusted life table mortality rates.
Health-Related Quality of Life and Health Care Costs
Quality of life (Qol) was related to HIV infection as well as the presence, severity, and treatment of obstructive CAD and the presence of CHF (appendix). Using the results of the EuroQol-5D questionnaire from the HIV-HEART study, we estimated the Qol for patients without severe CAD at 0.89.19 Qol of patients with cardiac diseases was derived by applying relative reductions in Qol as observed in the general population to the calculated health value. Patients who developed severe CAD were assumed to become fully symptomatic over the course of three years without cardiac treatment. Under treatment the Qol of patients with severe CAD was assumed to increase due to symptom relief; the recurrence of symptoms was reflected in a decline of Qol over time.15
Costs of diagnostic tests, cardiac interventions and cardiac medications were derived from German reimbursement catalogues and a previous health technology assessment (appendix). Costs associated with HIV infection and other diseases were estimated from the HIV-HEART study (appendix).
We adopted a societal perspective and reported all direct costs in 2007 Euros for Germany. Costs and quality-adjusted life years (QALYs) were discounted at 3%.
Results
Base-case Analysis
In a cohort of 1,000 HIV-positive men with at least an intermediate risk of CAD (10-year CAD risk ≥7.5%), stress-testing correctly identified 93 of 129 patients with severe CAD whereas CCTA correctly identified 117 of 129 patients with severe CAD.
In the “Stress-testing+Medication” strategy, 192 patients received a false positive result and were treated with cardiac medication for life; whereas in the “Stress-testing+Intervention” strategy these 192 patients underwent ICA. In the CCTA-based strategies 53 patients received a false positive CCTA result and either received lifetime medication in the “CCTA+Medication” strategy or underwent ICA in the “CCTA+Intervention” strategy.
All screening strategies were associated with additional diagnostic costs. The “Stress-testing+Medication” and “Functional-testing+Intervention” strategies resulted in additional costs of €73 and €579 per person whereas “CCTA+Medication” and “CCTA+Intervention” were associated with additional costs of €538 and €823, respectively.
The higher cost of the CCTA-based strategies relative to the stress-testing screening strategies can be explained by the increased cost of CCTA as compared to stress-ECG and stress-echocardiography [€500 vs. €31 vs. €89 (appendix)]. Moreover, the increased cost of screenings involving interventional diagnostics (ICA) were primarily caused by the greater number of ICAs performed [285 (“Stress-testing+Intervention”) vs. 19 (“Stress-testing+Medication) and 170 (“CCTA+Intervention”) vs. 20 (“CCTA+Medication”) per 1,000 patients].
Compared to no screening, the average life expectancy increased by 12 days in the “Stress-testing+Medication” strategy, 8 days in the “Stress-testing+Intervention” strategy, 16 days in the “CCTA+Medication” strategy and 10 days in the “CCTA+Intervention” strategy. These improvements correspond to 14, 11, 19, and 14 quality-adjusted life days, respectively. All strategies were associated with increased health care expenditures, resulting in additional costs per QALY gained. Incremental cost-effectiveness ratios (ICERs) relative to no screening are provided in Table 1.
Table 1.
Costs, Health Outcomes, and Cost-Effectiveness of One-Time CAD Screening Strategies Compared to “No Screening”
| Strategy | Cost (€) |
CAD/ CHF (€) |
HIV/ Other Diseases (€) |
QALYs | ICER (€/QALY) |
|---|---|---|---|---|---|
|
| |||||
| “No Screening” | 199,837 | 2,058 | 197,779 | 10.758 | |
|
| |||||
| “Stress-testing+Medication” | 201,752 | 3,712 | 198,040 | 10.797 | 49,261 |
| “Stress-testing+Intervention” | 201,618 | 3,618 | 198,000 | 10.789 | 57,817 |
|
| |||||
| “CCTA+Medication” | 201,620 | 3,480 | 198,140 | 10.809 | 34,887 |
| “CCTA+Intervention” | 202,081 | 4,110 | 197,971 | 10.798 | 56,518 |
, Euro; CAD, coronary artery disease; CHF, congestive heart failure; HIV, human immunodeficiency virus; QALYs, quality-adjusted life years; ICER, incremental cost-effectiveness ratio; CCTA, coronary computed tomography angiography. Deviations in ICERs due to rounding.
Note: Given that the chosen screening and intervention strategies depend more so on test availability and institutional preferences regarding management of CAD, we chose to compare each of the strategies to “no screening” as a reference strategy rather than conducting an incremental cost-effectiveness analysis.
Screening Threshold
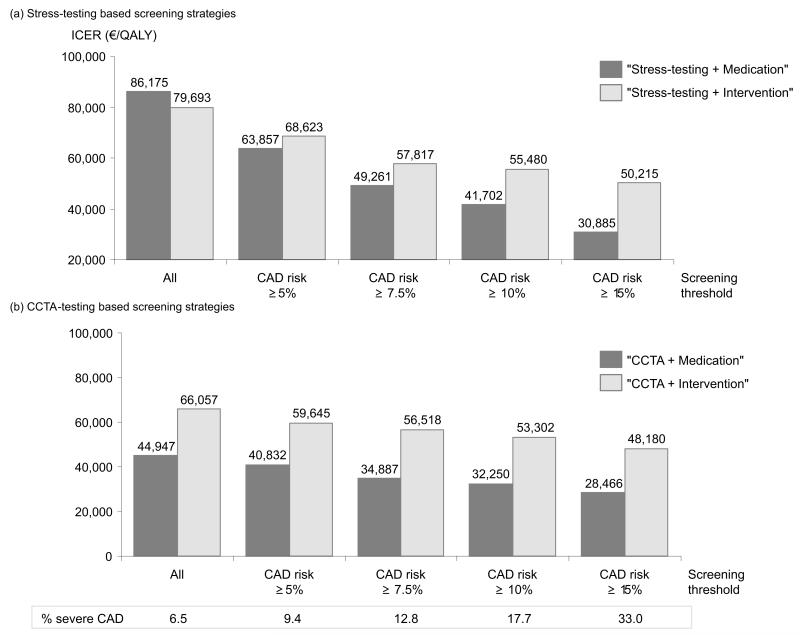
When varying the screening threshold criteria based on the Framingham risk score, we found screening strategies involving medical rather than interventional treatment to be the more cost-effective strategies compared to no screening for the majority of thresholds (figure 1). With increasing CAD prevalence, the ICERs of “Stress-testing+Medication” and “CCTA+Medication” compared to no screening decreased at higher rates than those of “Stress-testing+Intervention” and “CCTA+Intervention”; the ICERs of all strategies were lowest when setting the screening threshold at a 10-year CAD risk of >15%.
Figure 1.
Sensitivity analyses on the screening threshold
Screening Frequency
When screening frequency was varied, triennial “CCTA+Medication” resulted in the greatest gain in QALYs (0.069 QALYs) as compared to no screening; however, with an additional cost of €6,881 it was also the most expensive medication-based strategy resulting in an ICER of 552,294 €/QALY. Only the one-time screening strategies and “Stress-testing+Medication” every five years remained below the commonly applied willingness-to-pay threshold of 83,000 €/QALY (~100,000 US$/QALY in 2007; appendix).
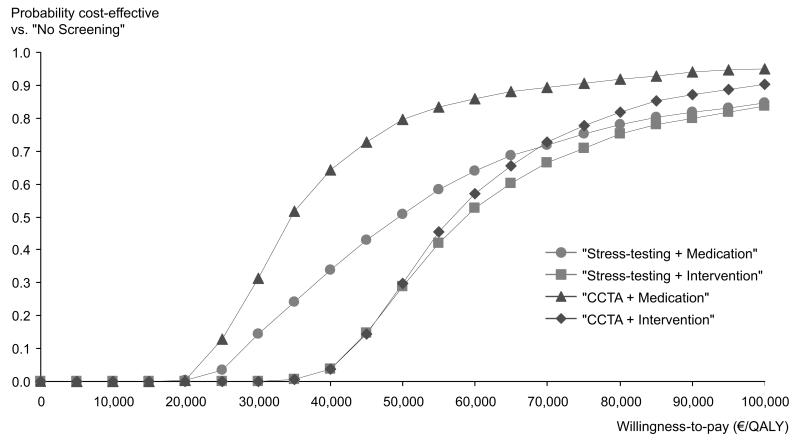
Probabilistic Sensitivity Analysis
Figure 1 displays the cost-effectiveness acceptability curves for all screening strategies compared to SOC. The graphs represent the probability of the different strategies being cost-effective, given a particular willingness-to-pay. At lower willingness-to-pay thresholds, SOC was most likely to be cost-effective, while with an increasing willingness-to-pay, the screening strategies became more likely to be cost-effective. At a willingness-to-pay threshold of 83,000 €/QALY, screening HIV-infected men with an intermediate CAD risk following the “Stress-testing+Medication” strategy was cost-effective with a probability of 79.3%. “Stress-testing+Intervention”, “CCTA+Medication” and “CCTA+Intervention” strategies were cost-effective with probabilities of 77%, 92.2% and 83.9%, respectively.
Discussion
Our analysis demonstrates that CAD screening in HIV-positive men without known cardiac disease may detect undiagnosed disease states, leading to a reduction in disease symptoms and increased quality-adjusted life expectancy (QALE). This gain in QALE compared to no screening is associated with additional costs both for screening and earlier treatment of CAD, resulting in €49,261, €57,817, €34,887 and €56,518 per QALY gained for the “Stress-testing+Medication”, “Stress-testing+Intervention”, “CCTA+Medication” and “CCTA+Intervention” strategies, respectively.
The cost-effectiveness of the screening strategies depended on the CAD prevalence and the imperfect test characteristics of stress-ECG and stress-echocardiography as well as CCTA. In a low risk population, the ratio between false positives and true positives was higher than in a population with a higher CAD prevalence, resulting in less favorable cost-effectiveness ratios. To bias our results against screening, we assumed that false positives received CAD treatment for life, thus accumulating CAD treatment costs without experiencing treatment benefit. Consequently, in a population with a low CAD risk, the cost per QALY gained was greater and the ICERs of “Stress-testing+Medication” (86,175 €/QALY) compared to SOC was marginally above the commonly applied willingness-to-pay threshold of 83,000 €/QALY (~100,000 US$/QALY in 2007). With increasing CAD prevalence which could be achieved in clinical practice by applying a pre-screening assessment, e.g., based on Framingham risk equations, the ICERs for all strategies compared to SOC decreased well below 83,000 €/QALY.
We assumed that CAD screening was performed in a previously unscreened population. Consequently, the detection of CAD cases in the initial one-time screening was higher than would be expected in routine periodic screenings. When assessing different screening intervals, we found all one-time screenings and “Stress-testing+Medication” every five years to be cost-effective.
To the best of our knowledge, this is the first cost-effectiveness analysis (CEA) of CAD screening in HIV-infected patients. Recent CEAs of the fusion inhibitor enfuvirtide and genotypic resistance testing for HAART optimization resulted in ICERs of 69,500 US$/QALY (73,024 €/QALY in 2007) and 69,600 €/QALY (66,028 €/QALY in 2007), respectively.20-21 These ICERs well above those of the assessed screening strategies and both interventions are currently recommended in Europe and the US.11-12,20-21 Only few CEAs of screenings for comorbidities in the HIV-infected population have been performed. Goldie et al reported ICERs of 13,000 US$/QALY (14,861 €/QALY in 2007 Euros) and 16,600 US$/QALY (18,977 €/QALY in 2007) for biennial and annual anal Papanicolaou testing.22
Few studies assessed the cost-effectiveness of CAD screening interventions in asymptomatic patients. Investigating alternative screening strategies for severe CAD in asymptomatic diabetic patients, Hayashino and colleagues found exercise echocardiography to be the dominant strategy, yielding gains of 0.095 and 0.193 QALYs as compared to no screening in cohorts of asymptomatic 55- and 60-year-old male diabetics.23 Based on the reported ICERs of 88,400 US$/QALY (87,004 €/QALY in 2007) for 55-year-old men and 40,800 US$/QALY (40,156 US$/QALY in 2007) for 60-year-old men, the authors concluded that CAD screening was acceptable from a societal perspective.23 Finally, in the context of other recommended screenings, such as breast cancer screening in women aged 50 to 74 years (ICER of 51,818 US$/QALY; 56,167 €/QALY in 2007), screening of HIV-positive men with at least an intermediate CAD risk can be considered cost-effective.24
There were several limitations to our analysis. First, the model does not consider smoking cessation as an intervention in addition to or in combination with medical therapy due to lack of available data. Given that smoking is a very prevalent modifiable risk factor in this population, cessation programs may be clinically beneficial and cost-effective.25-29 In addition, average excess mortality from HIV was modeled based on the initial distribution of CD4 cells in the HIV-HEART cohort.13 We chose not to model specific HIV-related infections since the focus of our analysis was to evaluate the impact of CAD screening in this population. It is reassuring that despite this choice our model predicted life expectancies consistent with those provided by the Antiretroviral Therapy Cohort Collaboration (ATCC) (36.6 year old men at HIV diagnosis would live to 65 years without screening in our model; 35-year old HIV-positive males on HAART were predicted to live up to 66.7 years in ATCC study).1
In all scenarios, we assumed 100% screening participation which may lead to an overestimation of screening benefits. However, HIV-infected patients are closely monitored by infectious disease and/or primary care physicians such that participation can be expected to be higher than in the general population (e.g., 60-70 % for mammography).30 Due to limited data on CAD prevalence in HIV-infected women, we exclusively evaluated CAD screening strategies in HIV-positive men. However, as reported by Triant and colleagues, HIV-positive women are at increased risk of cardiac events and may hence also benefit from CAD screening.4 Finally, while most of the clinical effectiveness predicted in this analysis can be directly generalized to the HIV-infected populations in other developed nations, there remains variability in costs among these health systems which may affect the generalizability of these results.
In conclusion, our analysis suggests that the incorporation of routine CAD screening into HIV treatment guidelines could improve health outcomes and be cost-effective. The ICERs of routine CAD screening in an HIV-infected male population without prior cardiac disease and a 10-year CAD risk ≥7.5% are below those of treatment interventions currently recommended in HIV-infected individuals.
Supplementary Material
Figure 2.
Cost-Effectiveness Acceptability Curve
Acknowledgments
Funding
This work was supported by the German Competence Network Heart Failure [stipend to J.E.H.N] and the National Institute of Health [Ruth Kirschstein National Research Award T32 to A.G., NIH R01 HL095123 to S.K.G., K24 DK 064545 to S.K.G, K23 HL 092792 to J.L.].
Footnotes
Declaration of Conflicting Interests
The Authors declare that there is no conflict of interest.
References
- 1.The Antiretroviral Therapy Cohort Collaboration Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet. 2008;372:293–299. doi: 10.1016/S0140-6736(08)61113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Currier JS, Taylor A, Boyd F, et al. Coronary heart disease in HIV-infected individuals. J Acquir Immune Defic Syndr. 2003;33:506–512. doi: 10.1097/00126334-200308010-00012. [DOI] [PubMed] [Google Scholar]
- 3.Law MG, Friis-Moller N, El-Sadr WM, et al. The use of the Framingham equation to predict myocardial infarctions in HIV-infected patients: comparison with observed events in the D:A:D Study. HIV Med. 2006;7:218–230. doi: 10.1111/j.1468-1293.2006.00362.x. [DOI] [PubMed] [Google Scholar]
- 4.Triant VA, Lee H, Hadigan C, et al. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92:2506–2512. doi: 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lo J, Abbara S, Shturman L, et al. Increased prevalence of subclinical coronary atherosclerosis detected by coronary computed tomography angiography in HIV-infected men. AIDS. 2010;24:243–253. doi: 10.1097/QAD.0b013e328333ea9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Currier JS, Lundgren JD, Carr A, et al. Epidemiological evidence for cardiovascular disease in HIV-infected patients and relationship to highly active antiretroviral therapy. Circulation. 2008;118:e29–35. doi: 10.1161/CIRCULATIONAHA.107.189624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Triant VA, Regan S, Lee H, et al. Association of immunologic and virologic factors with myocardial infarction rates in a US healthcare system. J Acquir Immune Defic Syndr. 2010;55:615–619. doi: 10.1097/QAI.0b013e3181f4b752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsue PY, Squires K, Bolger AF, et al. Screening and assessment of coronary heart disease in HIV-infected patients. Circulation. 2008;118:e41–47. doi: 10.1161/CIRCULATIONAHA.107.189626. [DOI] [PubMed] [Google Scholar]
- 9.Wilson PW, D’Agostino RB, Levy D, et al. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 10.Neumann T, Esser S, Potthoff A, et al. Prevalence and natural history of heart failure in outpatient HIV-infected subjects: rationale and design of the HIV-HEART study. Eur J Med Res. 2007;12:243–248. [PubMed] [Google Scholar]
- 11.Deutsche AIDS-Gesellschaft e.V., Österreichische AIDS Gesellschaft [accessed 26 December 2012];Deutsch-Österreichische Leitlinien zur antiretroviralen Therapie der HIV-1 Infektion. 2010 http://www.rki.de/DE/Content/InfAZ/H/HIVAIDS/Therapie/Leitlinien/D_A_antiretroviral_03_10.pdf?__blob=publicationFile.
- 12.Panel on Antiretroviral Guidelines for Adults and Adolescents [accessed 26 December 2012];Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. 2011 http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf.
- 13.Czoski-Murray C, Karnon J, Jones R, et al. [accessed 26 December 2012];Cost-effectiveness of screening high-risk HIV-positive men who have sex with men (MSM) and HIV-positive women for anal cancer. 2010 doi: 10.3310/hta14530. http://www.hta.ac.uk/fullmono/mon1453.pdf. [DOI] [PubMed]
- 14.Min JK, Lin FY, Dunning AM, et al. Incremental prognostic significance of left ventricular dysfunction to coronary artery disease detection by 64-detector row coronary computed tomographic angiography for the prediction of all-cause mortality: results from a two-centre study of 5330 patients. Eur Heart J. 2010;31:1212–1219. doi: 10.1093/eurheartj/ehq020. [DOI] [PubMed] [Google Scholar]
- 15.Ladapo JA, Jaffer FA, Hoffmann U, et al. Clinical outcomes and cost-effectiveness of coronary computed tomography angiography in the evaluation of patients with chest pain. J Am Coll Cardiol. 2009;54:2409–2422. doi: 10.1016/j.jacc.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 16.Ostrom MP, Gopal A, Ahmadi N, et al. Mortality incidence and the severity of coronary atherosclerosis assessed by computed tomography angiography. J Am Coll Cardiol. 2008;52:1335–1343. doi: 10.1016/j.jacc.2008.07.027. [DOI] [PubMed] [Google Scholar]
- 17.Kannel WB, D’Agostino RB, Silbershatz H, et al. Profile for estimating risk of heart failure. Arch Intern Med. 1999;159:1197–1204. doi: 10.1001/archinte.159.11.1197. [DOI] [PubMed] [Google Scholar]
- 18.Owan TE, Hodge DO, Herges RM, et al. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 19.Greiner W, Claes C, Busschbach JJ, et al. Validating the EQ-5D with time trade off for the German population. Eur J Health Econ. 2005;6:124–130. doi: 10.1007/s10198-004-0264-z. [DOI] [PubMed] [Google Scholar]
- 20.Sax PE, Losina E, Weinstein MC, et al. Cost-effectiveness of enfuvirtide in treatment-experienced patients with advanced HIV disease. J Acquir Immune Defic Syndr. 2005;39:69–77. doi: 10.1097/01.qai.0000160406.08924.a2. [DOI] [PubMed] [Google Scholar]
- 21.Yazdanpanah Y, Vray M, Meynard J, et al. The long-term benefits of genotypic resistance testing in patients with extensive prior antiretroviral therapy: a model-based approach. HIV Med. 2007;8:439–450. doi: 10.1111/j.1468-1293.2007.00491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldie SJ, Kuntz KM, Weinstein MC, et al. The clinical effectiveness and cost-effectiveness of screening for anal squamous intraepithelial lesions in homosexual and bisexual HIV-positive men. JAMA. 1999;281:1822–1829. doi: 10.1001/jama.281.19.1822. [DOI] [PubMed] [Google Scholar]
- 23.Hayashino Y, Nagata-Kobayashi S, Morimoto T, et al. Cost-effectiveness of screening for coronary artery disease in asymptomatic patients with Type 2 diabetes and additional atherogenic risk factors. J Gen Intern Med. 2004;19:1181–1191. doi: 10.1111/j.1525-1497.2004.40012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stout NK, Rosenberg MA, Trentham-Dietz A, et al. Retrospective cost-effectiveness analysis of screening mammography. J Natl Cancer Inst. 2006;98:774–782. doi: 10.1093/jnci/djj210. [DOI] [PubMed] [Google Scholar]
- 25.Critchley JA, Capewell S. Mortality risk reduction associated with smoking cessation in patients with coronary heart disease: a systematic review. JAMA. 2003;290:86–97. doi: 10.1001/jama.290.1.86. [DOI] [PubMed] [Google Scholar]
- 26.Quist-Paulsen P, Lydersen S, Bakke PS, et al. Cost effectiveness of a smoking cessation program in patients admitted for coronary heart disease. Eur J Cardiovasc Prev Rehabil. 2006;13:274–280. doi: 10.1097/01.hjr.0000192742.81231.91. [DOI] [PubMed] [Google Scholar]
- 27.Petoumenos K, Worm S, Reiss P, et al. Rates of cardiovascular disease following smoking cessation in patients with HIV infection: results from the D:A:D study(*) HIV Med. 2011;12:412–421. doi: 10.1111/j.1468-1293.2010.00901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reinsch N, Neuhaus K, Esser S, et al. Are HIV patients undertreated? Cardiovascular risk factors in HIV: results of the HIV-HEART study. Eur J Prev Cardiol. 2012;19:267–274. doi: 10.1177/1741826711398431. [DOI] [PubMed] [Google Scholar]
- 29.Wilson K, Hettle R, Marbaix S, et al. An economic evaluation based on a randomized placebo-controlled trial of varenicline in smokers with cardiovascular disease: results for Belgium, Spain, Portugal, and Italy. Eur J Prev Cardiol. 2012;19:1173–1183. doi: 10.1177/1741826711420345. [DOI] [PubMed] [Google Scholar]
- 30.Wallis M, Neilson F, Hogarth H, et al. Cumulative attendance, assessment and cancer detection rate over four screening rounds in five English breast-screening programmes: a retrospective study. J Public Health (Oxf) 2007;29:275–280. doi: 10.1093/pubmed/fdm020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.