Abstract
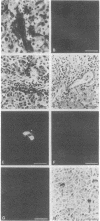
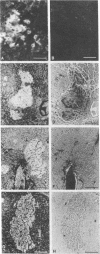
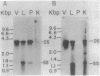
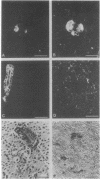
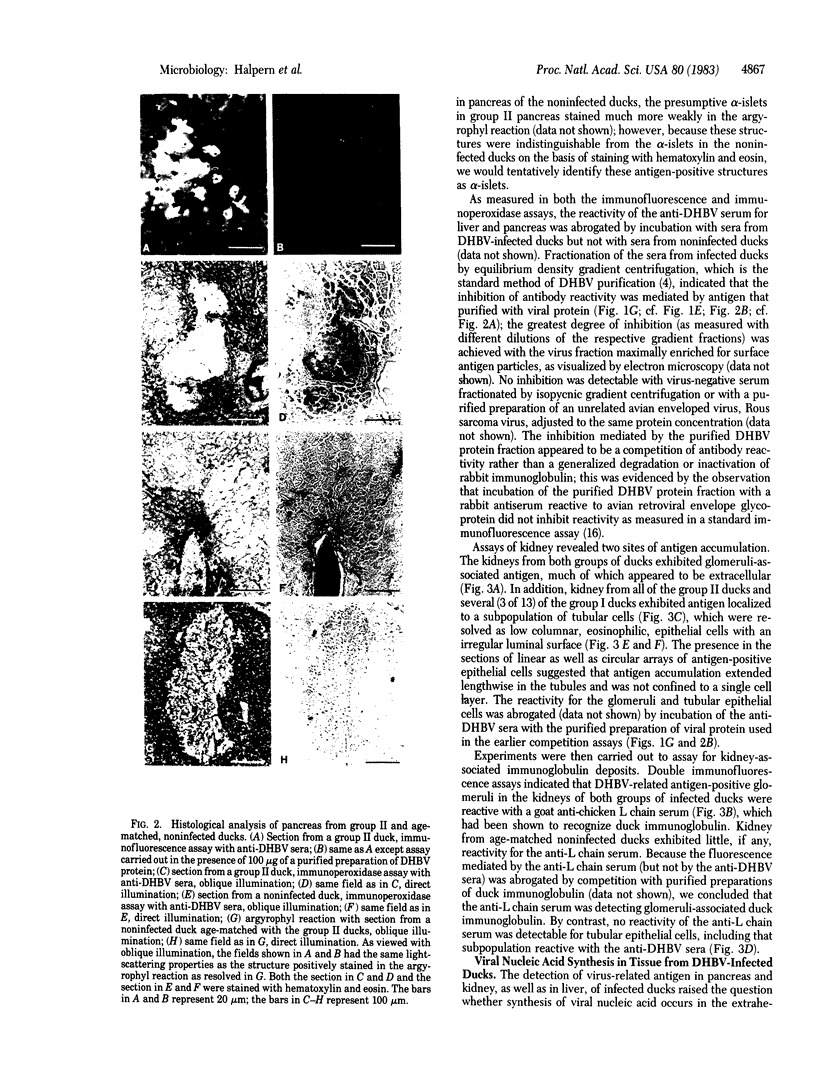
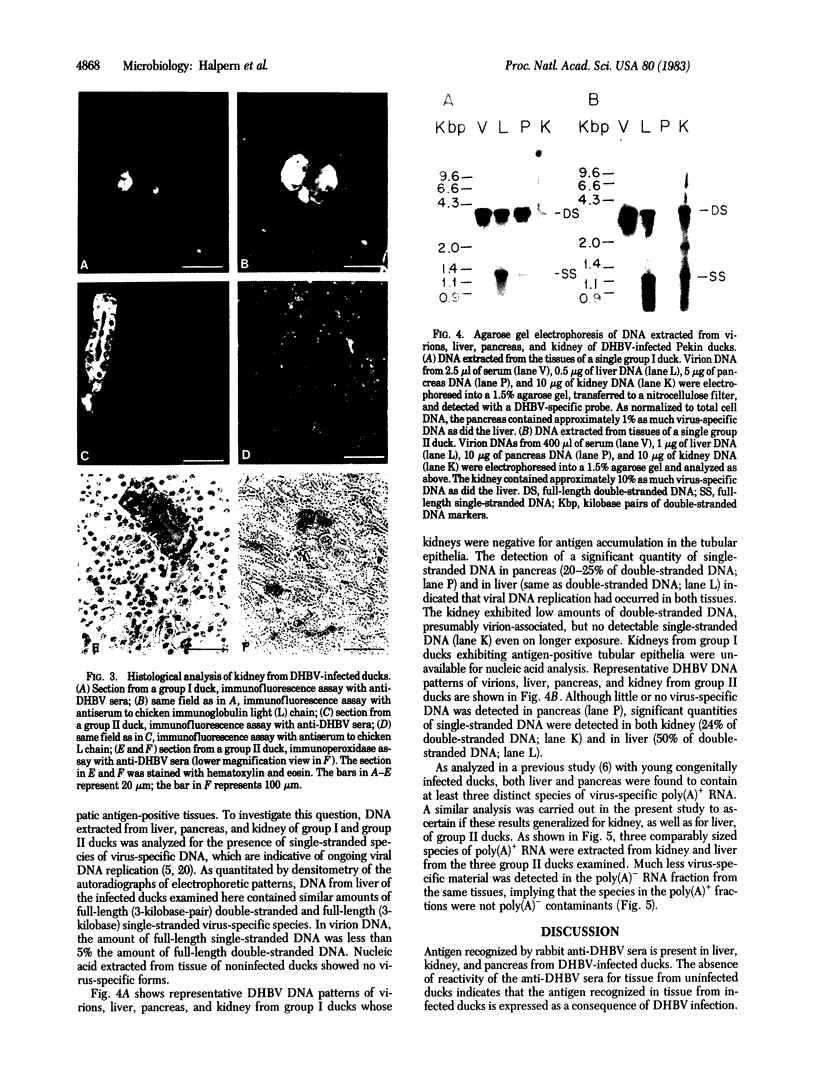
Liver, pancreas, and kidney from Pekin ducks infected with duck hepatitis B virus (DHBV) were assayed for the presence of both viral antigen and replication-specific forms of viral nucleic acid. In young congenitally infected ducks, antigen was detectable in hepatocytes and bile duct epithelia, in kidney glomeruli and tubular epithelia, and in cells localized to pancreatic acini. In older experimentally infected ducks, antigen was detectable in hepatocytes, in glomeruli and tubular epithelia, and in cells localized to presumptive pancreatic alpha-islets. All but the glomeruli-associated viral antigen appeared to be localized to the cytoplasm of antigen-positive cells. Much of the glomeruli-associated antigen appeared to be extracellular and was detected in glomeruli that were positive for the accumulation of immunoglobulin, observations suggestive of the deposition of viral antigen-antibody complexes. As analyzed with bulk tissue, replication-specific forms of viral nucleic acid were detectable in liver and pancreas from the young congenitally infected ducks and in liver and kidney from the older experimentally infected ducks.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brooks J. J. Immunohistochemistry of soft tissue tumors. Myoglobin as a tumor marker for rhabdomyosarcoma. Cancer. 1982 Nov 1;50(9):1757–1763. doi: 10.1002/1097-0142(19821101)50:9<1757::aid-cncr2820500919>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Coyne V. E., Millman I., Cerda J., Gerstley B. J., London T., Sutnick A., Blumberg B. S. The localization of Australia antigen by immunofluorescence. J Exp Med. 1970 Feb;131(2):307–319. doi: 10.1084/jem.131.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England J. M., Halpern M. S. Endogenous oncornaviral antigen in the bursa of Fabricius of 15B X 7(2) chickens. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2908–2911. doi: 10.1073/pnas.76.6.2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern M. S., Ewert D. L., Flores L. J., Lin K. Y., England J. M. Endogenous retroviral envelope antigen in plasma cells. J Immunol. 1981 Aug;127(2):698–702. [PubMed] [Google Scholar]
- Hoefs J. C., Renner I. G., Askhcavai M., Redeker A. G. Hepatitis B surface antigen in pancreatic and biliary secretions. Gastroenterology. 1980 Aug;79(2):191–194. [PubMed] [Google Scholar]
- Hsu T. W., Taylor J. M., Aldrich C., Townsend J. B., Seal G., Mason W. S. Tandem duplication of the proviral DNA in an avian sarcoma virus-transformed quail clone. J Virol. 1981 Apr;38(1):219–223. doi: 10.1128/jvi.38.1.219-223.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason W. S., Aldrich C., Summers J., Taylor J. M. Asymmetric replication of duck hepatitis B virus DNA in liver cells: Free minus-strand DNA. Proc Natl Acad Sci U S A. 1982 Jul;79(13):3997–4001. doi: 10.1073/pnas.79.13.3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason W. S., Seal G., Summers J. Virus of Pekin ducks with structural and biological relatedness to human hepatitis B virus. J Virol. 1980 Dec;36(3):829–836. doi: 10.1128/jvi.36.3.829-836.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoda T., Shikata T., Karasawa T., Tsukagoshi S., Yoshimura M., Sakurai I. Light microscopic localization of hepatitis B virus antigens in the human pancreas. Possibility of multiplication of hepatitis B virus in the human pancreas. Gastroenterology. 1981 Dec;81(6):998–1005. [PubMed] [Google Scholar]
- Smith G. E., Summers M. D. The bidirectional transfer of DNA and RNA to nitrocellulose or diazobenzyloxymethyl-paper. Anal Biochem. 1980 Nov 15;109(1):123–129. doi: 10.1016/0003-2697(80)90019-6. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Summers J., Mason W. S. Replication of the genome of a hepatitis B--like virus by reverse transcription of an RNA intermediate. Cell. 1982 Jun;29(2):403–415. doi: 10.1016/0092-8674(82)90157-x. [DOI] [PubMed] [Google Scholar]
- Taylor J. M., Illmensee R. Site on the RNA of an avian sarcoma virus at which primer is bound. J Virol. 1975 Sep;16(3):553–558. doi: 10.1128/jvi.16.3.553-558.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]