Abstract
Background:
Several studies have demonstrated considerable impairment of quality of life (QOL) in obstructive sleep apnea (OSA) patients, but its relation with severity of OSA is yet unclear.
Study Objectives:
To investigate the effects of OSA on the QOL and its association with the disease severity.
Design and Setting:
Observational, prospective case-control study.
Materials and Methods:
QOL of 69 OSA patients and 41 healthy controls were assessed using the Calgary sleep apnea quality of life index (SAQLI) on the morning following the polysomnography (PSG) study.
Statistics:
All statistical analyses were performed using the SPSS 17.0 (SPSS Inc., Chicago). Differences between sleep-related symptoms and SAQLI subscales scores were assessed with the Chi-square test and the Student t-test. Due to non-normal distribution, differences between SAQLI scores of controls and OSA patients were evaluated using a non-parametric Mann-Whitney test. Spearman correlation and backward multiple regression analysis were used to analyze the association between SAQLI scores and sleep indices and anthropometric variables and PSG variables.
Results:
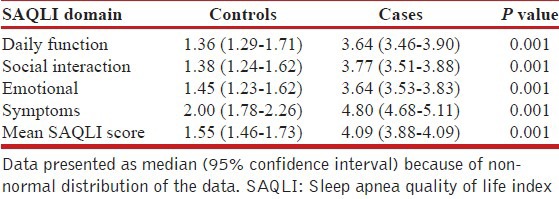
Study included 69 cases (57 male and 12 females) with a mean age, weight, height, neck circumference, and body mass index 48.45 ± 10.12 years, 83.03 ± 16.48 kg, 159.75 ± 28.29 cm, 44.01 ± 3.23 cm and 30.77 ± 6.71 kg/m2. Mean apnea-hypopnea index was 26.39 ± 16.62. The median score of four SAQLI domains daily function, social interaction, emotional, symptoms and total mean SAQLI score were 3.64 (3.46-3.90), 3.77 (3.51-3.88), 3.64 (3.53-3.83), 4.80 (4.68-5.11), 4.09 (3.88-4.09),and 1.36 (1.29-1.71), 1.38 (1.24-1.62), 1.45 (1.23-1.62), 2.00 (1.78-2.26), 1.55 (1.46-1.73) for patients and controls respectively. All the individual domain scores and the mean SAQLI scores of patients were significantly higher than the controls.
Conclusion:
OSA causes significant impairment of QOL, but the severity of impairment is not directly proportional to the severity of OSA.
KEY WORDS: Apnea, obstructive sleep, quality of life, sleep apnea quality of life index
INTRODUCTION
Obstructive sleep apnea (OSA) is characterized by the occurrence of daytime sleepiness, loud snoring, witnessed breathing interruptions, or awakenings due to gasping or choking in the presence of at least five obstructive respiratory events (apneas, hypopneas or respiratory effort related arousals) per hour of sleep. The presence of 15 or more obstructive respiratory events per hour of sleep in the absence of sleep related symptoms is also sufficient for the diagnosis of OSA due to the greater association of this severity of obstruction with important consequences such as increased cardiovascular disease risk. The combination of sleep-disordered breathing with daytime sleepiness is referred to as the OSA syndrome.[1] Prevalence surveys have estimated that about 4% of the middle-aged men and 2% of the middle-aged women are afflicted by OSA in developed countries.[2] Indian prevalence studies estimated disease prevalence rates of 3.5-13.7% (4.4-19.5% in males and 2.5-7.4% in females). Prevalence of OSAS in India is 1.7-3.6% (2.4-7.5% in males and 1-2.1% in females).[3,4,5,6]
The daytime sleepiness is the most common manifestation of OSA. However, other common daytime effects include irritability, decreased concentration, memory impairment, decreased energy and depressive symptoms.[7] Nocturnal symptoms include, restlessness, diaphoresis, awakenings with a sensation of choking or dyspnea, esophageal reflux, heartburn, laryngospasm, frequent nocturia, dry mouth etc. The most catastrophic result of excessive daytime sleepiness (EDS) is falling asleep behind the wheel and causing fatal automobile accidents.[8] Many studies have indicated an association between sleep apnea and cardiovascular/cerebrovascular related morbidity and mortality. It has been associated with the hypertension, coronary artery disease, congestive heart failure, arrhythmias and stroke.[9,10,11,12,13] It has also been associated with an increased mortality.[14]
Recently, the studies have confirmed that the impact of OSA on patient's quality of life (QOL) is rather more widespread than EDS, increased risk of cerebrovascular/cardiovascular events and other common features mentioned above. Certainly, there are many other domains of life, which remain unexplored in the sleep laboratory. In such cases, Flemons and Reimer and Lacasse et al. have outlined 4 such key domains of health related quality of life (HRQoL) viz. somatic sensation, physical function, emotional state, and social interaction.[15] As the measurements of physiological parameters alone cannot be taken surrogate markers of HRQoL, this emphasizes the need to measure QOL directly.[16]
Diverse self-reported instruments have been used to assess resulting impairment like medical outcomes study survey, Short-form 36 health survey Questionnaire (SF-36), Satisfaction with Life Scale, Nottingham Health Profile, General health questionnaire-28.[17,18,19,20,21] These HRQoL instruments have multiple subclasses, each signifying different domains of life.
Question mark on the ability of generic questionnaire to detect subtle effects of disease on QOL and effects on QOL brought about by various treatment modalities led to the development of disease specific questionnaire for OSA like Calgary sleep apnea quality of life index (SAQLI), functional outcome of sleepiness questionnaire (FOSQ), and OSA Patient-Oriented Severity Index.[22,23,24] These disease specific questionnaires have been compared with generic questionnaire. In a study, SAQLI was more responsive to changes in QOL in patients receiving OSA specific treatment than SF-36 and has strong content and constructive validity, but has to be administered by an interviewer.[16] These OSA specific questionnaires are being used increasingly in the newer studies and gradually replacing the generic scales.[25] A comparative study between these disease specific questionnaires have found SAQLI more sensitive than FOSQ.[26]
We undertook this study to investigate the overall effect of OSA on human life and where does it affect so that a complete therapy can be planned addressing the specific needs of the patient. Any treatment modality chosen on the basis of derangements in the physiological parameters only is unlikely to be complete as these parameters may not be the true representative of the extent of sufferings of the OSA patients. Thus, there is a need for a complete holistic treatment considering physiological, emotional, and social impairment of the individual patient.
The HRQoL has cross-cultural and regional differences even in same clinical condition.[27] Thus, the conclusions drawn from researches done outside India cannot be used to gauge QOL impairment in Indian OSA patients. This study was the need of the hour as there has not been any such study in India.
MATERIALS AND METHODS
The present study was conducted over a period of 18 months between March 2006 and August 2007, in an urban population in a North Indian City. The study was conducted after due approval from Ethics Committee of the Institute.
During the 1.5 year period consecutive adult patients of either sex who had been referred for snoring, EDS, multiple sleep awakenings were screened with Berlin Questionnaire. Those scored as high-risk cases were subjected to overnight Polysomnography (PSG) following clinical and lab evaluation. Patients with an apnea/hypopnea index ≥15 or ≥5 with symptoms of OSA were labeled as OSA cases. Those diagnosed as a case of OSA had their QOL assessed by SAQLI questionnaire. The original English version of the questionnaire developed by Flemons and Reimer was translated to the vernacular language of the patients.[22] The translated questionnaire was administered to each patient by the investigator, and the QOL was assessed by the ultimate score obtained by the patient.
Subjects who had experienced chronic psychiatric disorders, alcoholism, drug abuse, psychotropic drug abuse, unstable medical disorder, other sleep disorder were excluded. Similarly, severe respiratory disease (FEV1 <50% predicted), respiratory failure, hypothyroidism, or any illness, which could potentially bias perception of QOL, e.g., chronic obstructive lung diseases, bronchial asthma, heart failure, renal failure, depression etc., were excluded. The patients who were having stable medical conditions (e.g., Hypertension, Diabetes Mellitus) for more than 6 months were included in the study.
Controls
Age and gender matched controls were also assessed using the PSG and further subjected to SAQLI. Their SAQLI scores were compared with those of OSA patients. These control subjects were healthy volunteers, mostly attendants of patients admitted in our pulmonary medicine ward. Those with stable medical conditions were also included.
PSG
Each high-risk patient fulfilling inclusion criteria underwent whole night PSG using 44 channel Compumedics E-series-44 polysomnographic system. Parameters recorded were- EEG (Electroencephalogram), EOG (Electrooculogram), Chin and Leg EMG (Electromyogram), ECG (Electrocardiogram), Body positioning, thoracic and abdominal inductive respiratory Plethysmography, Pulse oximetry, airflow monitoring using the oronasal thermistors and snoring recording with the microphone. Sleep was scored in 30 s epochs while analysis and interpretation was performed according to standard criteria (R & K).[28]
Calculated sleep variables included total time on bed, total sleep time (TST), sleep latency, number of apneas, number of hypopneas, number of arousals (Arousals), number of arousals associated with the respiratory events (Respiratory Arousals) and RDI (Respiratory Disturbance Index). Apnea was defined as complete cessation of airflow for more than 10 s, hypopnea was defined as decrease in airflow of more than 50% with a desaturation of more than 4% for 10 s. Apnea-hypopnea index (AHI) was calculated as number of apneas and hypopneas per hour.
Calgary SAQLI
The SAQLI is a 35 item OSA specific QOL questionnaire having four main domains: daily functioning, social interactions, emotional functioning, and symptoms. In the first three domains, questions are framed to measure “how often” and “how much” symptoms interfere with daily activities, social interactions, and emotional state or are stated in degrees of frequency or difficulty and probe “how much of a problem” particular tasks are to perform. In the symptom domains, patients select the top five symptoms they experience related to OSA (from a list of 21). The questionnaire is administered by the interviewer.
A note about scoring
For each item of the SAQLI, patients were asked to give a graded subjective response. The degree of impairment/difficulty was rated on a 7-point Likert scale (1 to 7) ranging from not at all (1) to very large (7). One needs to reverse the response value for each of these items before summing for the total. That is, if the respondent gave a 1, you make it a 7; if they gave a 2 you make it a 6; 3 = 5; 4 = 4; 5 = 3; 6 = 2; and 7 = 1. These scores were then averaged for each domain by dividing by the number of questions in the domain. A total score was calculated by summing the four domains score and dividing by four, as has been described for the SAQLI.
Statistical analysis
All statistical analyses were performed using the SPSS 17.0 statistical program (SPSS Inc., Chicago, Ill., USA). Total as well as individual domains SAQLI scores of OSA patients and controls were tested for normal distribution of the data. Shapiro-Wilk test was applied for this analysis and values for total SAQLI scores were normally distributed among cases and controls (Sig. value 0.077 and 0.090 for cases and controls—value should be more than 0.05 for normal distribution). However, the individual SAQLI domains scores were mostly distributed non-normally. Thus, for the sake of uniformity values were considered to be distributed non-normally and SAQLI Scores were expressed as medians with 95% confidence intervals while polysomnographic and anthropometric values were expressed as means ± SD. Differences between sleep-related symptoms and subscales of the SAQLI scores were assessed using the Chi-square test and the Student t-test. Differences between controls and OSA patients were evaluated using a non-parametric test (Mann-Whitney U test). Association between the SAQLI scores and sleep indices were explored with the spearman correlation analysis. The correlation between the total score on the SAQLI, the anthropometric variables and PSG variables were determined by backward multiple regression analysis. P < 0.05 was considered significant.
RESULTS
Patient characteristics
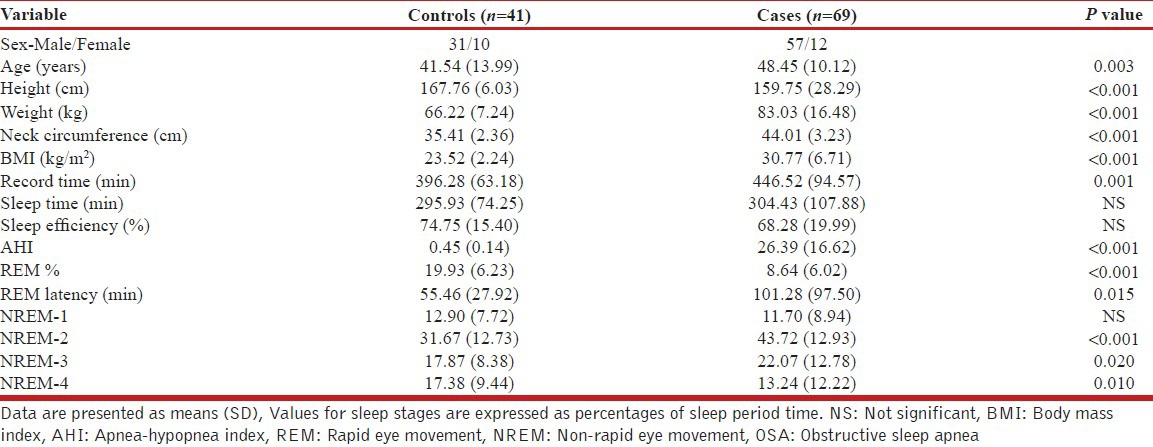
A total of 93 subjects were suspected of having OSA on the basis of a positive result of Berlin questionnaire. All suspected cases were subjected to overnight PSG study. PSG revealed 74 patients having confirmed OSA syndrome. Amongst 74 OSA patients, 2 were excluded as they were diagnosed to be suffering from Narcolepsy too. Two patients were found having hypothyroidism, hence excluded from the study. Another patient was having a history of significant depressive illness was also excluded from further evaluation. Finally, 69 OSA patients were included in our analysis out of which 22 patients had mild, 21 moderate, and 26 suffered severe OSA. Out of these 69 patients, 12 (17.4%) were females and rest 57 (82.6%) were males. 43 (62.3%) patients were graduate/postgraduate, two patients (2.9%) were uneducated and rest 24 (34.7%) were school pass-outs. 47 (68.1%) patients gave a history of snoring while history of EDS, Multiple sleep awakenings and sleepiness while driving was present in 42 (60.8%), 29 (42%) and 17 (24.6%) patients respectively. 39 patients (56.5%) patients were hypertensive, 23 (33.3%) were diabetics and family history of OSA was present in 14 (20.3%) cases. Fourty-one normal healthy controls were also included in the study. Table 1 shows mean ± SD values for anthropometric, clinical and polysomnographic parameters among the analyzed patients and controls. The two groups were significantly different in age, height, weight, body mass index and neck circumference. As far as polysomnographical parameters are concerned, Table 1 reveals statistically significant differences between the groups. The OSA group had higher Recording time, AHI, rapid eye movement (REM) latency, non-rapid eye movement (NREM)-2 and 3% and lower REM sleep percentage and NREM-4%.
Table 1.
Clinical characteristics and polysomnography results of the OSA patients and controls
SAQLI score
SAQLI scores of patients and controls are given in Table 2. All the individual domain scores and the mean SAQLI scores of patients were significantly higher than the controls suggesting poorer QOL.
Table 2.
Comparison of the SAQLI score of the patients and controls
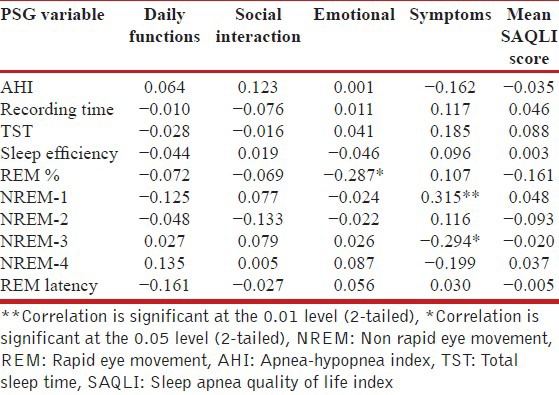
Correlation of QOL and severity of OSA
The association between the AHI and the SAQLI scores was explored with spearman correlation analysis as the data were non-parametric. Among the cases of OSA, mean SAQLI score (r = −0.035, P = 0.774) and symptoms domain score (r = −0.162, P = 0.185) showed non-significant negative correlation with AHI. Daily function (r = 0.064, P = 0.603), social Interaction (r = 0.123, P = 0.315) and emotional (r = 0.001, P = 0.992) domains scores had non-significant positive correlation with AHI. Thus there was no significant correlation of severity of OSA with severity of QOL impairment.
QOL and polysomnographic variables
Table 3 shows the correlation of SAQLI Score and polysomnographic variables. The emotional domain of SAQLI statistically significant negative correlation with percentage of REM sleep (r = −0.287, P < 0.05) while symptoms domain score showed significant positive correlation with NREM-1 sleep (r = 0.315, P < 0.01) and negative correlation with NREM-3 (r = −0.294, P < 0.05).
Table 3.
Spearman's correlation coefficient between SAQLI scores, and polysomnography variables of OSA cases
DISCUSSION
We studied the QOL in patients of OSA and observed that QOL was impaired in patients of OSA in our study. All the 4 domains of SAQLI scale viz.; daily activities, emotional functioning, social interactions, and symptoms were significantly impaired in OSA cases when compared to that of controls. Daily functioning, social interaction and emotional functioning had almost equal impairment, but the symptoms domain was worst affected which is on expected lines as these 5 most affected symptoms were chosen by patients themselves. It was observed that all aspects of life covered under SAQLI scale were affected in the disease state. Somewhat similar results have been observed by many researchers across different parts of the globe.
Although study by Lacasse et al. revealed much lower scores of all four domains viz., “daily life activities”; 1.0, “social interactions”; 1.0, “emotions”; 0.7, and “symptoms”; 2.5, but here too symptoms domain was the one which was most affected.[16] D’Ambrosio et al. used SF-36 for measuring QOL and observed that all dimensions of the QOL were significantly impaired in patients of OSA as compared to the normal population.[29] Akashiba et al. observed that 6 of the eight domains score of the SF-36 scale and the overall total SF-36 scale score was significantly lower than that in the control subjects. The two domains viz. physical functioning and body pain were not found affected. This could be explained on the basis that SF-36 questionnaire is not specific for OSA and such generic questionnaires are likely to have domains which are not affected by a specific disease.[18] Fornas et al. administered Nottingham Health Profile questionnaire to measure QOL and observed that OSA patients showed deterioration of general health status parameters in comparison to healthy subjects[21] Gall et al. using SF-36 observed that patients with OSA have significant impaired social functioning[30] Finn et al. studied self-reported general health status in sleep-disordered breathing cases in Wisconsin Sleep Cohort Study. Even mild sleep-disordered breathing of apnea-hypopnea index of 5, was associated with decrement in SF-36.[31] These results are especially notable because they come from a population-based sample. However, it is worth emphasizing here that in this study, the SF-36 was self-administered by sending to all participants through the postal service and was completed about 3 years after the diagnosis by PSG. Hence, the bias due to such a long gap cannot be ruled out. Manocchia et al. observed a direct association between sleep problems and decrement in HRQoL in OSAS cases as measured by SF-36 scale[17] but their patients also had other co-morbid conditions, which could have caused sleepiness/other effects and hence these results may not be truly due to OSAS alone. The study by Moore et al. suggested that RDI and number of arousals were significantly associated with mobility, cognitive functioning, social functioning, energy, fatigue, and health distress. There was also significant correlation between pain severity and RDI, number of arousals or arousals with respiratory events. Interestingly patients had been well screened and were not suffering from severe or even moderate levels of pain.[32] Pain complaints of any kind are not generally thought to be part of the clinical picture in OSA. Author explained that though not directly a feature of OSA, pain is an important part of the symptom complex in other disorders that may include disturbed sleep such as fibromyalgia. Therefore, this may be taken as another evidence of redundancy and inadequacy of generic questionnaires. Sforza et al. discovered that all HRQoL dimensions were decreased in sleep related breathing disorder patients, with a greater impact on SF-36 sub scores for “vitality,” “physical role,” “social functioning,” “mental health,” and “role emotional dimensions.”[33] Several other studies also found QOL impaired in one or more domains.[34]
Some other studies observed there is no impairment of QOL in patients of OSA. G³ebocka et al., didn’t find any significant psychological disturbances in patients of OSA as compared to normal healthy adults.[20] Author attributed rather unexpected lack of psychological differences to the rapid mood improvement in OSA patients on anticipation of being diagnosed and taken care of in the hospital setting.
In our study, we observed that the QOL impairment was not in direct proportion with severity of OSA disease. Some patients with mild OSA were having QOL impairment equivalent or even more than what was observed in patients with severe OSA. Conversely, some patients with severe OSA were found having only minimal impairment of QOL. Many other studies have observed similar results.[34,30]
Our study is having some limitations, which might have affected the results. (1) The cases may not be the true representative of the population as females were under represented as compared to population prevalence (2) Original SAQLI questionnaire is written in English; it may not be equally suitable in other language and cultures. However, studies have shown that SAQLI has been used in other cultures and languages quite successfully.[35] (3) Our sampling was directly not from community as suspected patients coming to hospital were enrolled in the study, although, patients were selected randomly. (4) The patients in the community might be having disease stages different than to those who reported to hospital. (5) We used a translated version of the SAQLI questionnaire which was not validated. The true morbidity of OSA might be more than this because of little awareness of OSA in India. Population based studies are required to gauge the sufferings of OSA patients in the community.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005. American Academy of Sleep Medicine. International Classification of Sleep Disorders: Diagnostic and Coding Manual. [Google Scholar]
- 2.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 3.Sharma SK, Kumpawat S, Banga A, Goel A. Prevalence and risk factors of obstructive sleep apnea syndrome in a population of Delhi, India. Chest. 2006;130:149–56. doi: 10.1378/chest.130.1.149. [DOI] [PubMed] [Google Scholar]
- 4.Udwadia ZF, Doshi AV, Lonkar SG, Singh CI. Prevalence of sleep-disordered breathing and sleep apnea in middle-aged urban Indian men. Am J Respir Crit Care Med. 2004;169:168–73. doi: 10.1164/rccm.200302-265OC. [DOI] [PubMed] [Google Scholar]
- 5.Vijayan VK, Patial K. Prevalence of obstructive sleep apnea syndrome (OSAS) in Delhi, India. Chest. 2006;130:92S–c. [Google Scholar]
- 6.Reddy EV, Kadhiravan T, Mishra HK, Sreenivas V, Handa KK, Sinha S, et al. Prevalence and risk factors of obstructive sleep apnea among middle-aged urban Indians: A community-based study. Sleep Med. 2009;10:913–8. doi: 10.1016/j.sleep.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 7.Cheshire K, Engleman H, Deary I, Shapiro C, Douglas NJ. Factors impairing daytime performance in patients with sleep apnea/hypopnea syndrome. Arch Intern Med. 1992;152:538–41. [PubMed] [Google Scholar]
- 8.Terán-Santos J, Jiménez-Gómez A, Cordero-Guevara J. The association between sleep apnea and the risk of traffic accidents. Cooperative Group Burgos-Santander. N Engl J Med. 1999;340:847–51. doi: 10.1056/NEJM199903183401104. [DOI] [PubMed] [Google Scholar]
- 9.Shahar E, Whitney CW, Redline S, Lee ET, Newman AB, Nieto FJ, et al. Sleep-disordered breathing and cardiovascular disease: Cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 10.Franklin KA, Nilsson JB, Sahlin C, Näslund U. Sleep apnoea and nocturnal angina. Lancet. 1995;345:1085–7. doi: 10.1016/s0140-6736(95)90820-x. [DOI] [PubMed] [Google Scholar]
- 11.Mehra R, Benjamin EJ, Shahar E, Gottlieb DJ, Nawabit R, Kirchner HL, et al. Association of nocturnal arrhythmias with sleep-disordered breathing: The Sleep Heart Health Study. Am J Respir Crit Care Med. 2006;173:910–6. doi: 10.1164/rccm.200509-1442OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khayat RN, Jarjoura D, Patt B, Yamokoski T, Abraham WT. In-hospital testing for sleep-disordered breathing in hospitalized patients with decompensated heart failure: Report of prevalence and patient characteristics. J Card Fail. 2009;15:739–46. doi: 10.1016/j.cardfail.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dyken ME, Im KB. Obstructive sleep apnea and stroke. Chest. 2009;136:1668–77. doi: 10.1378/chest.08-1512. [DOI] [PubMed] [Google Scholar]
- 14.Young T, Finn L, Peppard PE, Szklo-Coxe M, Austin D, Nieto FJ, et al. Sleep disordered breathing and mortality: Eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31:1071–8. [PMC free article] [PubMed] [Google Scholar]
- 15.Lacasse Y, Godbout C, Sériès F. Health-related quality of life in obstructive sleep apnoea. Eur Respir J. 2002;19:499–503. doi: 10.1183/09031936.02.00216902. [DOI] [PubMed] [Google Scholar]
- 16.Lacasse Y, Godbout C, Sériès F. Independent validation of the Sleep Apnoea Quality of Life Index. Thorax. 2002;57:483–8. doi: 10.1136/thorax.57.6.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manocchia M, Keller S, Ware JE. Sleep problems, health-related quality of life, work functioning and health care utilization among the chronically ill. Qual Life Res. 2001;10:331–45. doi: 10.1023/a:1012299519637. [DOI] [PubMed] [Google Scholar]
- 18.Akashiba T, Kawahara S, Akahoshi T, Omori C, Saito O, Majima T, et al. Relationship between quality of life and mood or depression in patients with severe obstructive sleep apnea syndrome. Chest. 2002;122:861–5. doi: 10.1378/chest.122.3.861. [DOI] [PubMed] [Google Scholar]
- 19.Goncalves MA, Paiva T, Ramos E, Guilleminault C. Obstructive sleep apnea syndrome, sleepiness, and quality of life. Chest. 2004;125:2091–6. doi: 10.1378/chest.125.6.2091. [DOI] [PubMed] [Google Scholar]
- 20.G³ebocka A, Kossowska A, Bednarek M. Obstructive sleep apnea and the quality of life. J Physiol Pharmacol. 2006;57(Suppl 4):111–7. [PubMed] [Google Scholar]
- 21.Fornas C, Ballester E, Arteta E, Ricou C, Diaz A, Fernandez A, et al. Measurement of general health status in obstructive sleep apnea hypopnea patients. Sleep. 1995;18:876–9. doi: 10.1093/sleep/18.10.876. [DOI] [PubMed] [Google Scholar]
- 22.Flemons WW, Reimer MA. Development of a disease-specific health-related quality of life questionnaire for sleep apnea. Am J Respir Crit Care Med. 1998;158:494–503. doi: 10.1164/ajrccm.158.2.9712036. [DOI] [PubMed] [Google Scholar]
- 23.Weaver TE, Laizner AM, Evans LK, Maislin G, Chugh DK, Lyon K, et al. An instrument to measure functional status outcomes for disorders of excessive sleepiness. Sleep. 1997;20:835–43. [PubMed] [Google Scholar]
- 24.Piccirillo JF. Outcomes research and obstructive sleep apnea. Laryngoscope. 2000;110(3 Pt 3):16–20. doi: 10.1097/00005537-200003002-00005. [DOI] [PubMed] [Google Scholar]
- 25.Stucki A, Cieza A, Schuurmans MM, Ustun B, Stucki G, Gradinger F, et al. Content comparison of health-related quality of life instruments for obstructive sleep apnea. Sleep Med. 2008;9:199–206. doi: 10.1016/j.sleep.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 26.Kasibowska-Kuźniar K, Jankowska R, Kuźniar T, Brzecka A, Piesiak P, Zwierzycki J. Comparative evaluation of two health-related quality of life questionnaires in patients with sleep apnea. Wiad Lek. 2004;57:229–32. [PubMed] [Google Scholar]
- 27.Buck D, Jacoby A, Baker GA, Ley H, Steen N. Cross-cultural differences in health-related quality of life of people with epilepsy: Findings from a European study. Qual Life Res. 1999;8:675–85. doi: 10.1023/a:1008916326411. [DOI] [PubMed] [Google Scholar]
- 28.Kales A, Rechtschaffen A. University of California LABIS, NINDB Neurological Information Network (U. S.). A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. In: Rechtschaffen A, Kales A, editors. Bethesda, Md: U. S. National Institute of Neurological Diseases and Blindness Neurological Information Network; 1968. [Google Scholar]
- 29.D’Ambrosio C, Bowman T, Mohsenin V. Quality of life in patients with obstructive sleep apnea: Effect of nasal continuous positive airway pressure—A prospective study. Chest. 1999;115:123–9. doi: 10.1378/chest.115.1.123. [DOI] [PubMed] [Google Scholar]
- 30.Gall R, Isaac L, Kryger M. Quality of life in mild obstructive sleep apnea. Sleep. 1993;16(Suppl 8):S59–61. doi: 10.1093/sleep/16.suppl_8.s59. [DOI] [PubMed] [Google Scholar]
- 31.Finn L, Young T, Palta M, Fryback DG. Sleep-disordered breathing and self-reported general health status in the Wisconsin Sleep Cohort Study. Sleep. 1998;21:701–6. [PubMed] [Google Scholar]
- 32.Moore P, Bardwell WA, Ancoli-Israel S, Dimsdale JE. Association between polysomnographic sleep measures and health-related quality of life in obstructive sleep apnea. J Sleep Res. 2001;10:303–8. doi: 10.1046/j.1365-2869.2001.00264.x. [DOI] [PubMed] [Google Scholar]
- 33.Sforza E, Janssens JP, Rochat T, Ibanez V. Determinants of altered quality of life in patients with sleep-related breathing disorders. Eur Respir J. 2003;21:682–7. doi: 10.1183/09031936.03.00087303. [DOI] [PubMed] [Google Scholar]
- 34.Yang EH, Hla KM, McHorney CA, Havighurst T, Badr MS, Weber S. Sleep apnea and quality of life. Sleep. 2000;23:535–41. [PubMed] [Google Scholar]
- 35.Mok WY, Lam CL, Lam B, Cheung MT, Yam L, Ip MS. A Chinese version of the Sleep Apnea Quality of Life Index was evaluated for reliability, validity, and responsiveness. J Clin Epidemiol. 2004;57:470–8. doi: 10.1016/j.jclinepi.2003.09.018. [DOI] [PubMed] [Google Scholar]


