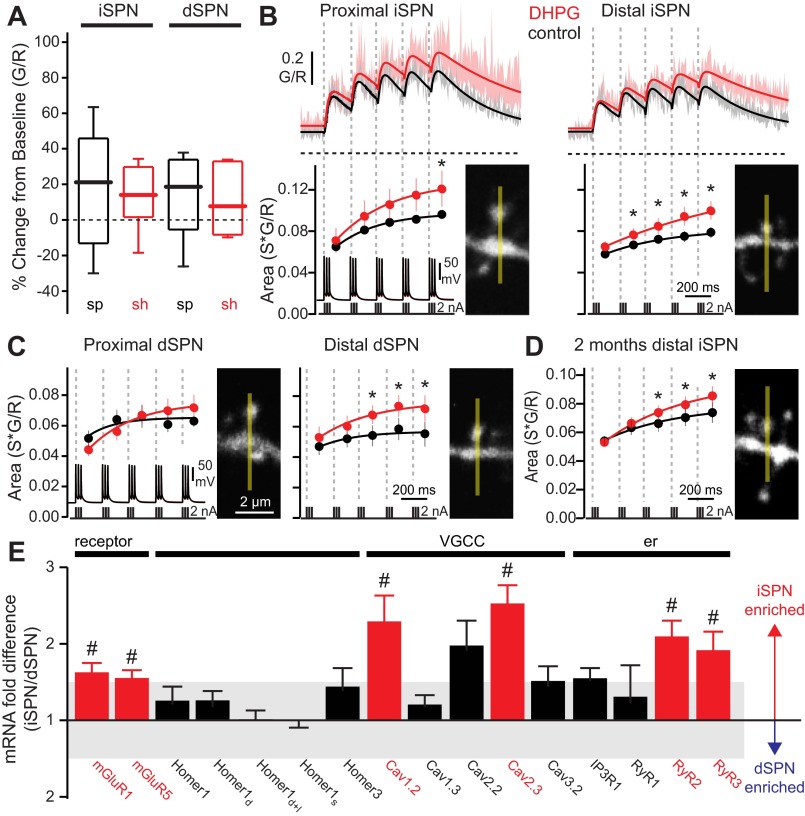
Fig. 3.
Activation of group I metabotropic glutamate receptors (mGluRs) enhances bAP-evoked Ca2+ transients in iSPN and dSPN dendritic spines. A: box plots showing basal intracellular Ca2+ concentrations in distal (100–120 μm from soma) spines (sp) and shafts (sh) of iSPNs and dSPNs, before and after bath application of (S)-3,5-dihydroxyphenylglycine (DHPG, 50 μM; n = 5 cells, 8–10 spines, 7–9 shafts). DHPG itself had no significant effect on dendritic basal Ca2+. B: average theta burst bAP-evoked Ca2+ transients in iSPN dendritic spines before and after bath application of DHPG (50 μM). Shaded areas indicate SE; solid lines are summating biexponential fits of the average G/R transients. Dashed horizontal lines indicate G/R = 0. Ca2+ transient areas corresponding to each burst are shown at bottom (temporal boundaries indicated by dashed lines; dots are averages, bars are SE). Ca2+ transient areas are fit with a single exponential. DHPG significantly increased Ca2+ transients in iSPN spines (proximal: n = 5 cells, 10 spines; distal: n = 5 cells, 11 spines). C: as in B, for dSPNs (proximal: n = 4 cells, 6 spines; distal: n = 5 cells, 9 spines). DHPG significantly increased Ca2+ transients in distal dSPN spines. D: average theta burst bAP-evoked Ca2+ transient areas in distal dendritic spines of 2-mo-old iSPNs (n = 4 cells, 24 spines). DHPG significantly increased Ca2+ transients in mature iSPN spines. *P < 0.05, Wilcoxon signed-rank test. E: relative mRNA expression in FACS-pooled dSPNs and iSPNs (n = 4–17), showing cell-specific differential regulation of membrane-, scaffold-, and endoplasmic reticulum (er)-associated genes. VGCC, voltage-gated Ca2+ channel. #P < 0.05, 2-tailed t-test.