Table 1.
Thiourea catalyst optimization
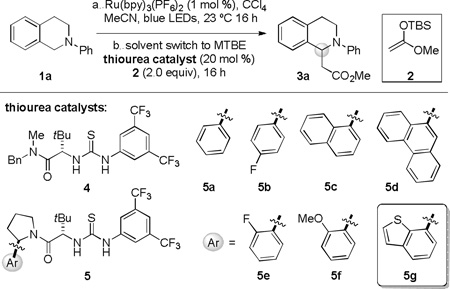 | |||||
|---|---|---|---|---|---|
| entry | thiourea catalyst |
T (°C)a | [1a]o (M) | yield[%]b | ee[%]c |
| 1 | - | −78 | 0.1 | 0 | N/A |
| 2 | 4 | −78 | 0.1 | 75 | 10 |
| 3 | 5a | −78 | 0.1 | 68 | 50 |
| 4 | 5b | −78 | 0.1 | 63 | 20 |
| 5 | 5c | −78 | 0.1 | 57 | 80 |
| 6 | 5d | −78 | 0.1 | 63 | 29 |
| 7 | 5c | −60 | 0.05 | 37 | 93 |
| 8 | epi-5c d | −60 | 0.05 | 68 | 44 |
| 9 | 5e | −60 | 0.05 | 63 | 87 |
| 10 | 5f | −60 | 0.05 | 63 | 85 |
| 11 | ent-5g | −60 | 0.05 | 72 | −97 |
Reaction temperature for the alkylation step in MTBE.
Yields determined by 1H NMR spectroscopic analysis of the crude reaction mixture relative to 2,5-dimethylfuran as the internal standard.
Determined by HPLC on commercial chiral columns.
Pyrrolodine configuration is (R), tert-leucine configuration is (S).
