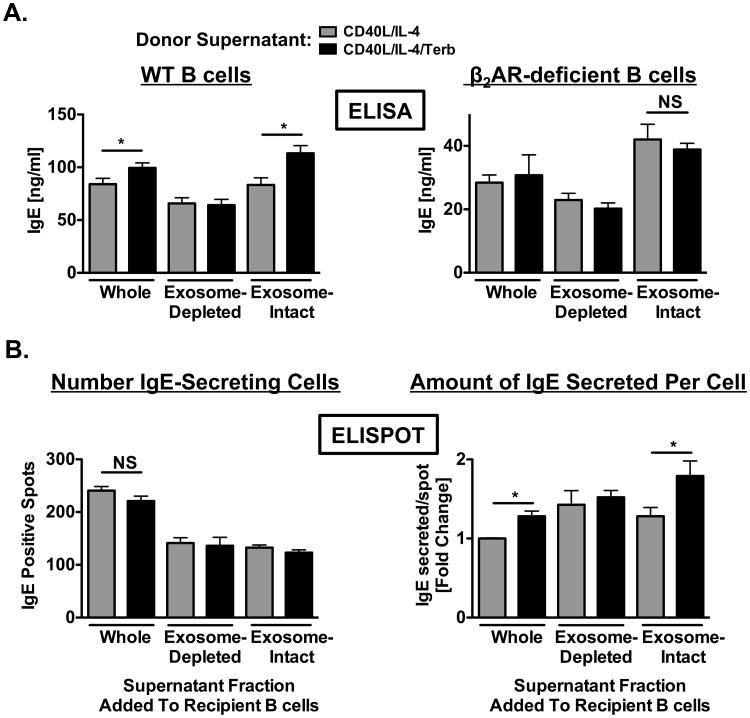
Figure 10.
Transfer of exosomes derived from primed B cells exposed to a β2AR agonist directly enhance IgE production by recipient B cells. Donor supernatants: resting B cells were cultured as described in Figure 1 in the presence (black) or absence (grey) of terbutaline. Supernatants containing B cell-derived exosomes were removed after two days and the supernatant was fractionated by ultracentrifugation into exosome-intact and exosome-depleted components. Recipient B cells: resting B cells were cultured as described in Figure 1 in the absence of terbutaline. Whole, exosome-intact, or exosome-depleted supernatants were transferred in the presence of IL-4 (1 ng/ml) to recipient cells that were primed for 2 days in the absence of terbutaline and which had supernatants removed prior to donor supernatant transfers. (A) Supernatant fractions were transferred from donor cultures containing either WT (left panel) or β2AR-deficient (right panel) B cells. IgE produced by recipient cells was measured by ELISA. Data are presented as mean IgE values (ng/ml) +/- SEM from one representative independent experiment of three from at least three replicate measurements. (B) Comparison of the number of recipient IgE-secreting cells (left panel) and the amount of IgE secreted by these cells (right panel) using the ELISPOT assay. Data are presented as the number of IgE-positive spots/well or as the fold change compared to whole supernatant transfer in IgE secreted on a per spot basis +/- SEM from one representative independent experiment of three from at least three replicate measurements. Significance is based on an unpaired t-test. *, p = .05, as compared to B cells exposed to fractions obtained from primed B cells alone.