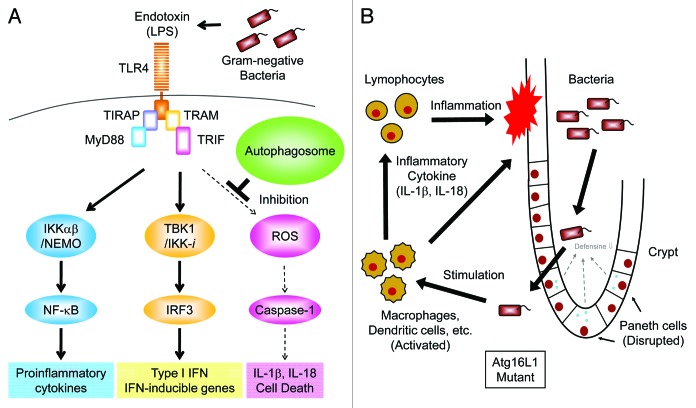
Figure 2. Autophagy suppresses endotoxin-induced inflammatory immune responses. (A) TLR4 triggers both MyD88- and TRIF-dependent signaling pathways in response to LPS. The IKKα-IKKβ-NEMO complex mediates the activation of the transcription factor NFκB which, in turn, induces transcription of pro-inflammatory cytokines, such as TNFα, IL-6 and pro-IL-1β. The TBK1-IKK-i complex mediates the activation of the transcription factor IRF-3, which then induces the transcription of Type I IFN and IFN-inducible genes. In autophagy-deficient cells, high levels of ROS are generated and mediate TRIF-dependent caspase-1 activation resulting in the production of IL-1β. However, in wild-type macrophages, limited amounts of IL-1β are induced by LPS due to a lack of ROS generation. (B) Hypothetical model of intestinal inflammation caused by autophagy deficiency. The intestinal epithelial layer lacking autophagy seems to be susceptible to microbial infection due to the disrupted maturation of Paneth cells. The barrier function of intestinal epithelial cells is also compromised by chemical stress such as DSS exposure resulting in the invasion of commensal bacteria. Macrophages and dendritic cells detect invading bacteria and produce inflammatory cytokines. Autophagy-deficient macrophages produce high levels of IL-1β and IL-18 leading to severe inflammation mediated by lymphocytes.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.