Abstract
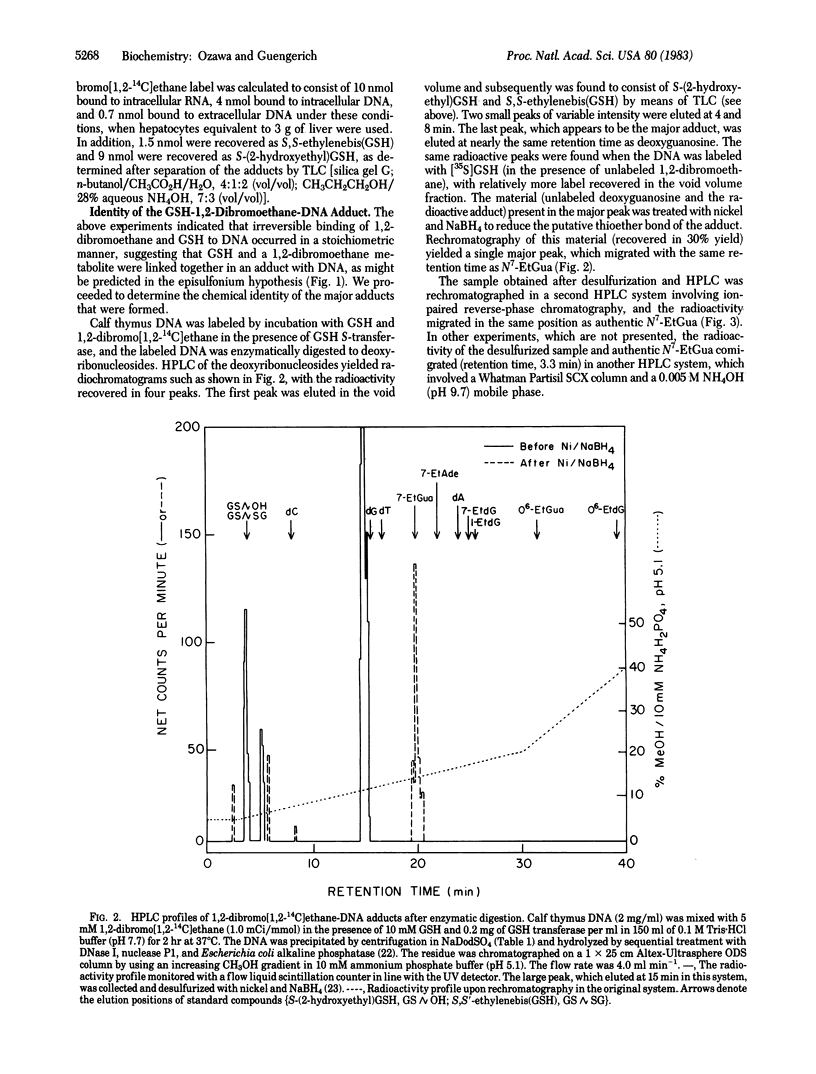
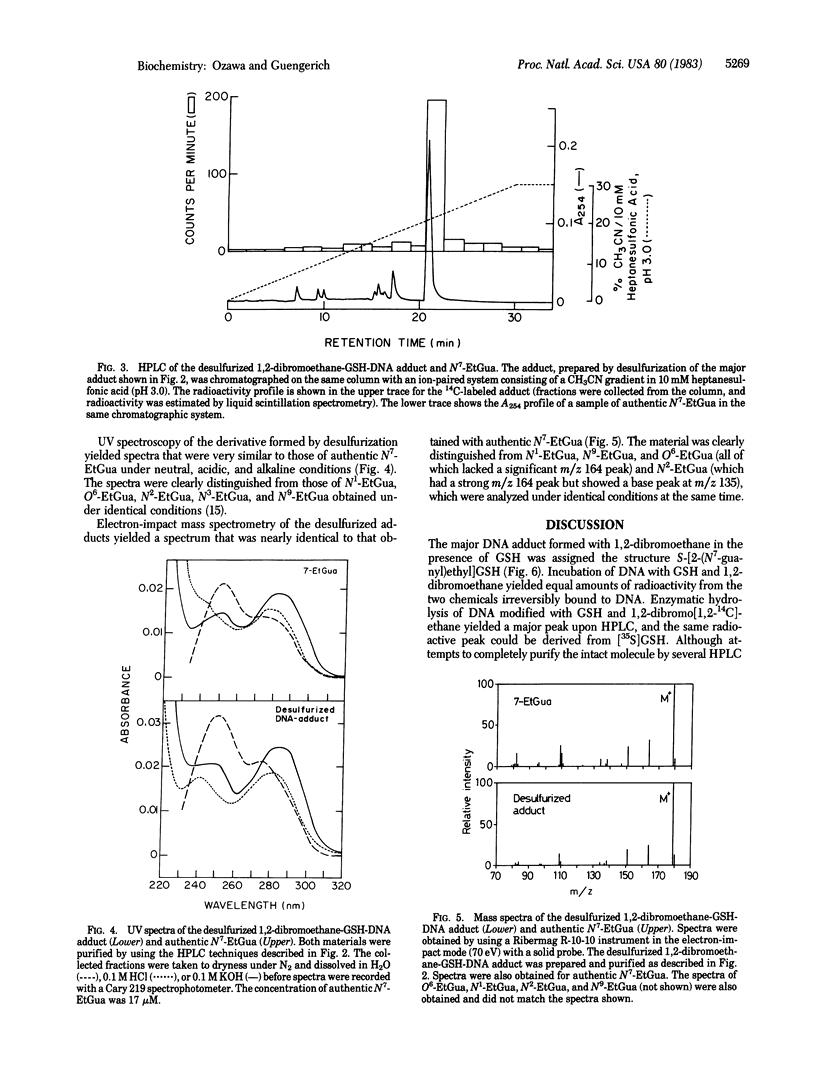
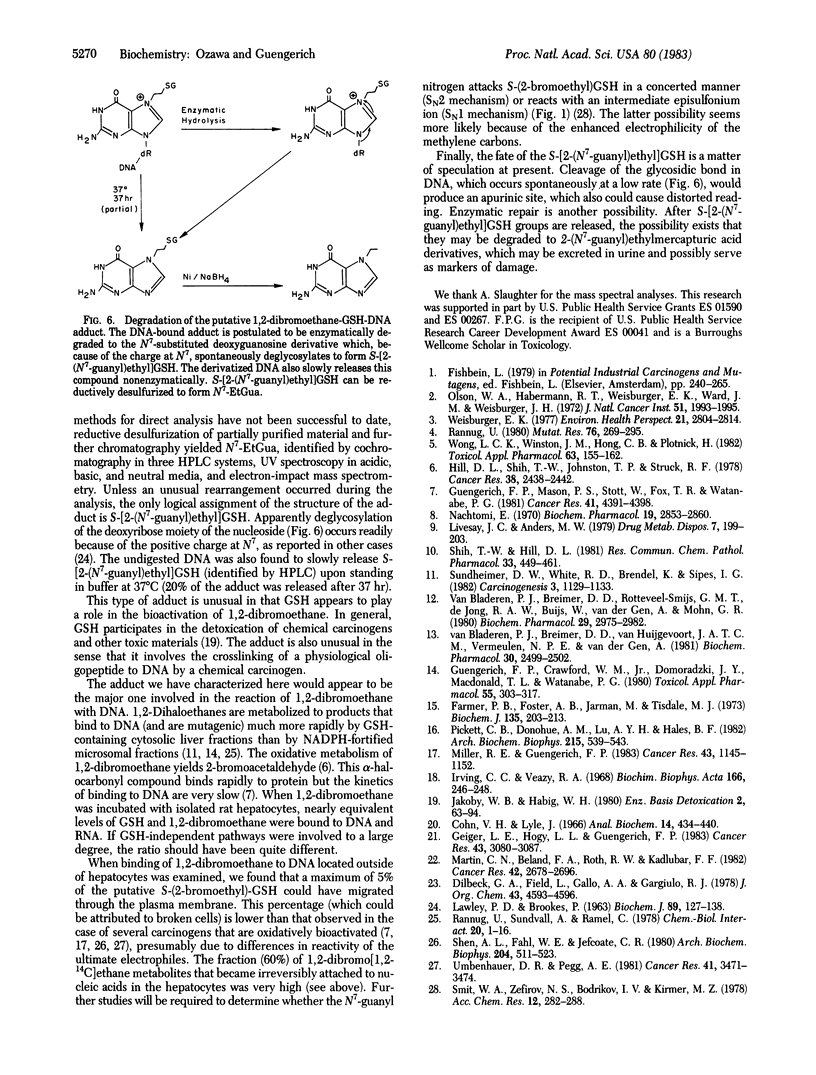
The carcinogen 1,2-dibromoethane and reduced glutathione (GSH) were irreversibly bound to calf thymus DNA in equimolar amounts when in vitro incubations were carried out in the presence of GSH S-transferase. In studies carried out with isolated hepatocytes, equimolar amounts of 1,2-dibromoethane and endogenous GSH were also bound to intracellular DNA and RNA and extracellular DNA. These findings support the hypothesis that the major interaction of 1,2-dibromoethane with DNA involves covalent modification by a preformed complex of the carcinogen and GSH--i.e., S-(2-bromoethyl)GSH or the resulting episulfonium ion. Enzymatic hydrolysis of calf thymus DNA labeled with 1,2-dibromoethane in the presence of GSH and GSH S-transferase and subsequent high-performance liquid chromatography of the residues yielded a major fraction, which also was found to contain radiolabel derived from GSH. The fraction thus isolated was reductively desulfurized to yield N7-ethylguanine, which was isolated and identified by comparison with authentic material in two other high-performance liquid chromatography systems and by UV and mass spectrometry. Therefore, the structure of the undesulfurized adduct is assigned as S-[2-(N7-guanyl)ethyl]GSH. This adduct is unusual in that it is involved in a situation in which GSH plays a role in the bioactivation of a chemical carcinogen, as opposed to the more typical detoxication reactions. Further, a chemical carcinogen has been shown to cross-link DNA with a small physiological peptide.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cohn V. H., Lyle J. A fluorometric assay for glutathione. Anal Biochem. 1966 Mar;14(3):434–440. doi: 10.1016/0003-2697(66)90286-7. [DOI] [PubMed] [Google Scholar]
- Farmer P. B., Foster A. B., Jarman M., Tisdale M. J. The alkylation of 2'-deoxyguanosine and of thymidine with diazoalkanes. Some observations on o-alkylation. Biochem J. 1973 Sep;135(1):203–213. doi: 10.1042/bj1350203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger L. E., Hogy L. L., Guengerich F. P. Metabolism of acrylonitrile by isolated rat hepatocytes. Cancer Res. 1983 Jul;43(7):3080–3087. [PubMed] [Google Scholar]
- Guengerich F. P., Crawford W. M., Jr, Domoradzki J. Y., Macdonald T. L., Watanabe P. G. In vitro activation of 1,2-dichloroethane by microsomal and cytosolic enzymes. Toxicol Appl Pharmacol. 1980 Sep 15;55(2):303–317. doi: 10.1016/0041-008x(80)90092-7. [DOI] [PubMed] [Google Scholar]
- Guengerich F. P., Mason P. S., Stott W. T., Fox T. R., Watanabe P. G. Roles of 2-haloethylene oxides and 2-haloacetaldehydes derived from vinyl bromide and vinyl chloride in irreversible binding to protein and DNA. Cancer Res. 1981 Nov;41(11 Pt 1):4391–4398. [PubMed] [Google Scholar]
- Hill D. L., Shih T. W., Johnston T. P., Struck R. F. Macromolecular binding and metabolism of the carcinogen 1,2-dibromoethane. Cancer Res. 1978 Aug;38(8):2438–2442. [PubMed] [Google Scholar]
- Irving C. C., Veazey R. A. Isolation of deoxyribonucleic acid and ribosomal ribonucleic acid from rat liver. Biochim Biophys Acta. 1968 Aug 23;166(1):246–248. doi: 10.1016/0005-2787(68)90508-x. [DOI] [PubMed] [Google Scholar]
- LAWLEY P. D., BROOKES P. FURTHER STUDIES ON THE ALKYLATION OF NUCLEIC ACIDS AND THEIR CONSTITUENT NUCLEOTIDES. Biochem J. 1963 Oct;89:127–138. doi: 10.1042/bj0890127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livesey J. C., Anders M. W. In vitro metabolism of 1,2-dihaloethanes to ethylene. Drug Metab Dispos. 1979 Jul-Aug;7(4):199–203. [PubMed] [Google Scholar]
- Martin C. N., Beland F. A., Roth R. W., Kadlubar F. F. Covalent binding of benzidine and N-acetylbenzidine to DNA at the C-8 atom of deoxyguanosine in vivo and in vitro. Cancer Res. 1982 Jul;42(7):2678–2686. [PubMed] [Google Scholar]
- Miller R. E., Guengerich F. P. Metabolism of trichloroethylene in isolated hepatocytes, microsomes, and reconstituted enzyme systems containing cytochrome P-450. Cancer Res. 1983 Mar;43(3):1145–1152. [PubMed] [Google Scholar]
- Nachtomi E. The metabolism of ethylene dibromide in the rat: the enzymic reaction with glutathione in vitro and in vivo. Biochem Pharmacol. 1970 Nov;19(11):2853–2860. doi: 10.1016/0006-2952(70)90024-9. [DOI] [PubMed] [Google Scholar]
- Olson W. A., Habermann R. T., Weisburger E. K., Ward J. M., Weisburger J. H. Induction of stomach cancer in rats and mice by halogenated aliphatic fumigants. J Natl Cancer Inst. 1973 Dec;51(6):1993–1995. doi: 10.1093/jnci/51.6.1993. [DOI] [PubMed] [Google Scholar]
- Pickett C. B., Donohue A. M., Lu A. Y., Hales B. F. Rat liver glutathione S-transferase B: the functional mRNAs specific for the Ya Yc subunits are induced differentially by phenobarbital. Arch Biochem Biophys. 1982 May;215(2):539–543. doi: 10.1016/0003-9861(82)90113-8. [DOI] [PubMed] [Google Scholar]
- Rannug U. Genotoxic effects of 1,2-dibromoethane and 1,2-dichloroethane. Mutat Res. 1980 Nov;76(3):269–295. doi: 10.1016/0165-1110(80)90020-2. [DOI] [PubMed] [Google Scholar]
- Rannug U., Sundvall A., Ramel C. The mutagenic effect of 1,2-dichloroethane on Salmonella typhimurium I. Activation through conjugation with glutathion in vitro. Chem Biol Interact. 1978 Jan;20(1):1–16. doi: 10.1016/0009-2797(78)90076-5. [DOI] [PubMed] [Google Scholar]
- Shen A. L., Fahl W. E., Jefcoate C. R. Metabolism of benzo(a)pyrene by isolated hepatocytes and factors affecting covalent binding of benzo(a)pyrene metabolites to DNA in hepatocyte and microsomal systems. Arch Biochem Biophys. 1980 Oct 15;204(2):511–523. doi: 10.1016/0003-9861(80)90063-6. [DOI] [PubMed] [Google Scholar]
- Shih T. W., Hill D. L. Metabolic activation of 1,2-dibromoethane by glutathione transferase and by microsomal mixed function oxidase: further evidence for formation of two reactive metabolites. Res Commun Chem Pathol Pharmacol. 1981 Sep;33(3):449–461. [PubMed] [Google Scholar]
- Sundheimer D. W., White R. D., Brendel K., Sipes I. G. The bioactivation of 1,2-dibromoethane in rat hepatocytes: covalent binding to nucleic acids. Carcinogenesis. 1982;3(10):1129–1133. doi: 10.1093/carcin/3.10.1129. [DOI] [PubMed] [Google Scholar]
- Umbenhauer D. R., Pegg A. E. Alkylation of intracellular and extracellular DNA by dimethylnitrosamine following activation by isolated rat hepatocytes. Cancer Res. 1981 Sep;41(9 Pt 1):3471–3474. [PubMed] [Google Scholar]
- Wong L. C., Winston J. M., Hong C. B., Plotnick H. Carcinogenicity and toxicity of 1,2-dibromoethane in the rat. Toxicol Appl Pharmacol. 1982 Apr;63(2):155–165. doi: 10.1016/0041-008x(82)90036-9. [DOI] [PubMed] [Google Scholar]
- van Bladeren P. J., Breimer D. D., Rotteveel-Smijs G. M., de Jong R. A., Buijs W., van der Gen A., Mohn G. R. The role of glutathione conjugation in the mutagenicity of 1,2-dibromoethane. Biochem Pharmacol. 1980 Nov 1;29(21):2975–2982. doi: 10.1016/0006-2952(80)90047-7. [DOI] [PubMed] [Google Scholar]


