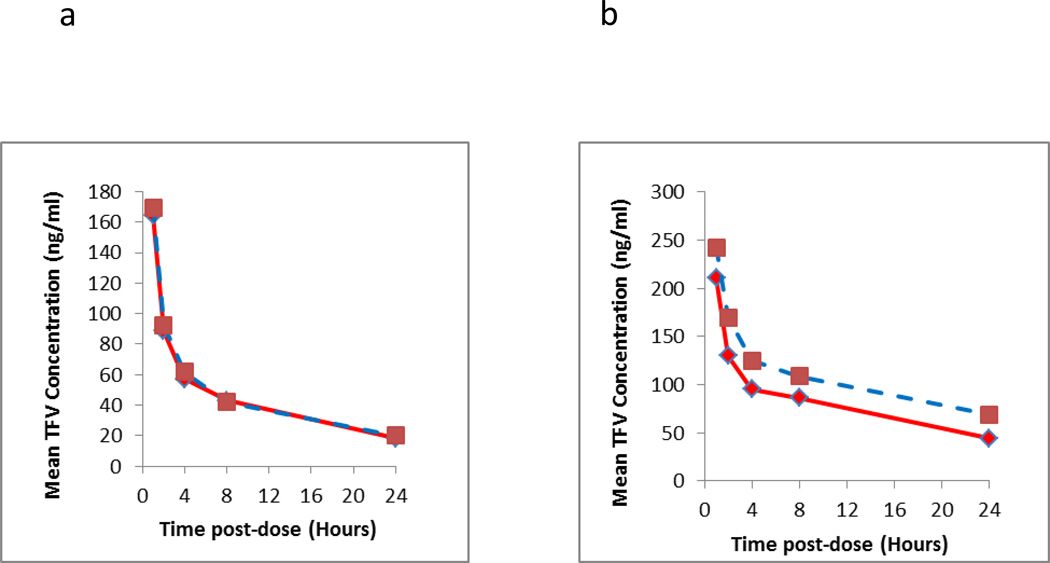
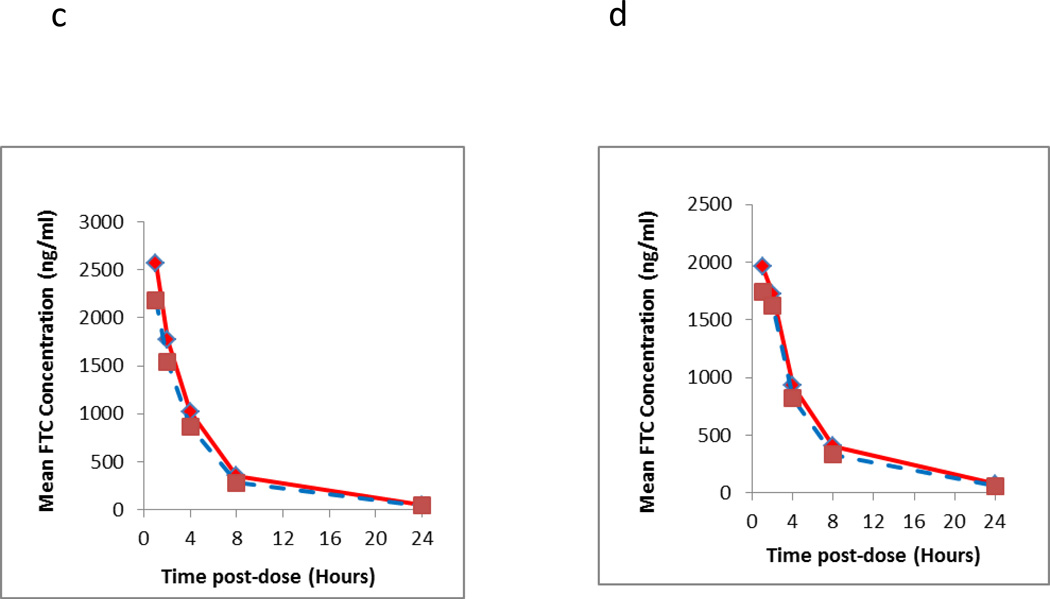
Figure 3.
Intensive pharmacokinetic results evaluating the long term storage stability of clinical samples at room temperature from two different dosing days (first dose, i.e. Day 1 and Day 30). The Y axis represents the mean of measured TFV (3a Day 1;3b Day 30) and FTC (3c Day 1;3d Day 30) of three subjects at the respective time point and dosing days from the initial run (solid line) and re-run (dashed line). The elapsed time between two runs was 404 days.