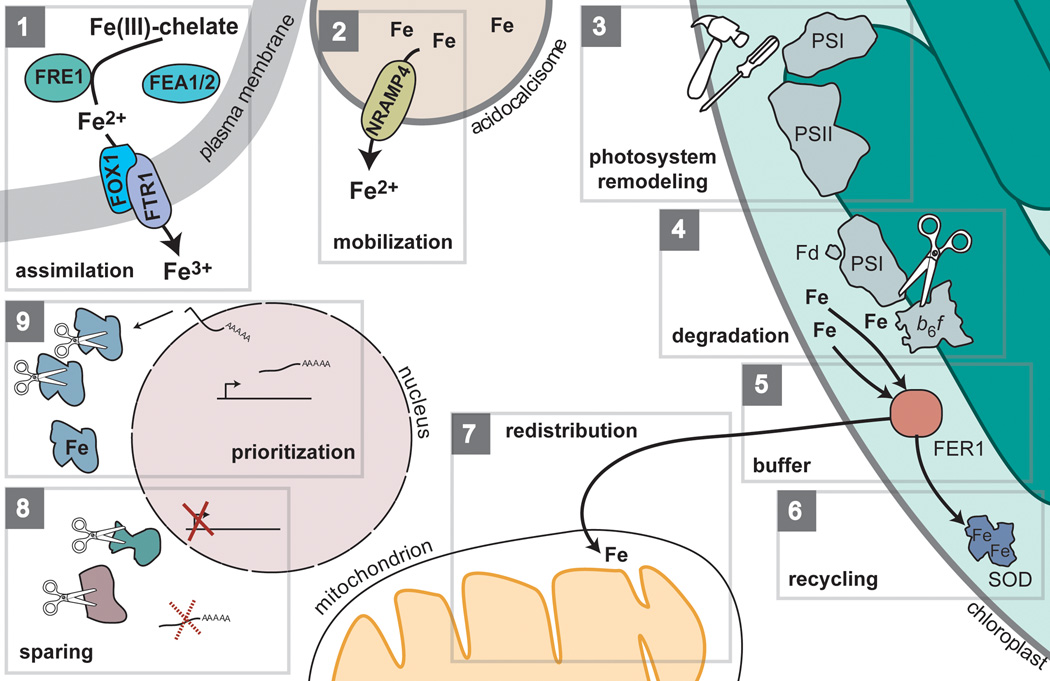
Figure 2.
Chlamydomonas and suboptimal iron nutrition during photoheterotrophic growth. (1) The first response is to induce the expression of the FOX1/FTR1 high-affinity iron transporter, which is composed of a copper-dependent ferroxidase (FOX1) and an iron permease (FTR1). Accompanying this transporter is a ferrireductase FRE1 and the algal-specific proteins FEA1 and FEA2, which are also proposed to participate in iron assimilation. (2) Assimilation may be combined with iron mobilization from vacuole-like compartments called acidocalcisomes. NRAMP4, whose expression is specifically induced by iron deficiency, is orthologous to the vacuole-localized Arabidopsis iron transporter NRAMP4, but its function in Chlamydomonas has not been established experimentally. (3) When, despite the operation of these two pathways, poor iron nutrition prevents cells from meeting the iron quota, the light harvesting antenna associated with photosystem I (PSI) and photosystem II (PSII) are remodeled. Specifically, the light harvesting antenna associated with PSI is disconnected at the onset of iron deficiency followed by degradation and replacement of some light-harvesting subunits [15], whereas the functional PSII antenna size increases [16]. This process is thought to reduce photooxidative stress caused by the photoreduction of dioxygen to superoxide by PSI. Superoxide destroys solvent exposed Fe-S clusters (including potentially those from PSI) releasing Fe3+. This unchelated iron can then react with hydrogen peroxide creating the highly cytotoxic hydroxyl radical, which cannot be enzymatically destroyed. (4) The loss of iron-dependent components, specifically Cyt b6f, PSI and ferredoxin, may proceed through active degradation of iron-bound proteins. The released iron may be temporarily chelated by ferritin (5) before it is recycled to other proteins (6) such as the Fe-dependent superoxide dismutase in the chloroplast. (7) Additionally, maintenance of oxygen consumption by the mitochondria points to redistribution of this iron pool for preservation of oxidative phosphorylation [16]. To further ensure optimal iron use, (8) the abundance of dispensable iron-dependent proteins is markedly reduced, possibly either through repression of expression, transcript degradation, or degradation of apoprotein. (9) The abundance of indispensible iron-dependent proteins is maintained (although sometimes at a lower abundance as in the case of the ferredoxin FDX6 [17]) by induction of gene expression and/or transcript stability.