Abstract
A phase I-II study to evaluate gene mediated cytotoxic immunotherapy in newly diagnosed prostate cancer before radical prostatectomy was conducted in Monterrey, Mexico.
Methods
To investigate delivery of adenovirus to the prostate, fluorescently labeled vector was injected into fresh prostatectomy specimens and distribution visually analyzed. The optimal volume and site instillation was then used for transrectal ultrasound guided intraprostatic injection in 10 patients with adenocarcinoma scheduled for radical prostatectomy. Each received 2-apical and 2-basal 0.5 ml injections of AdV-tk for a total of 1×1011 vp followed by 14 days of prodrug. Nine patients continued to tumor resection: 6 high-risk, 1 intermediate and 2 low-risk. In-vivo vector distribution was analyzed from resected tissue of four patients. Patients were monitored for tumor progression and acute and long-term safety.
Results
Two apical and two basal injections of 0.5ml led to optimal organ-wide distribution of an adenoviral vector ex-vivo and in-vivo. Cytotoxicity was evidenced by transient rise in PSA and tumor histology. There were no significant adverse events deemed related to the treatment and no late toxicities after median follow up of 11.3 years. All six high-risk patients had positive surgical margins and one had seminal vesicle involvement. Despite slow PSA rise post-surgery in 3 of these patients, none developed metastases. The intermediate and low-risk patients had complete resections and none have progressed.
Conclusion
In-vivo transrectal ultrasound guided instillation of an adenoviral vector into four sites in the prostate was practical as an outpatient procedure, well tolerated and led to distribution throughout the intraprostatic tumor mass. AdV-tk demonstrated no significant acute or late toxicities. Trends in PSA and disease progression conveyed the possibility of a sustained immune response against residual disease.
Keywords: Prostate cancer, Gene-Mediated Cytotoxic Immunotherapy, intra-tumor vector distribution, long-term safety, androgen deprivation therapy
Introduction
Prostate cancer is second only to lung as a cause of death due to cancer in men in Mexico and worldwide 1,2. In many Latin American and other developing countries, screening for early prostate cancer detection is not widely applied. Thus, many patients present with advanced disease 3. The incidence of prostate cancer is expected to increase as health-care administration improves and life expectancy increases in these developing nations. Standard of care for localized prostate cancer consists of radical prostatectomy (RP) or radiation therapy (RT) with adjuvant androgen deprivation therapy for higher risk patients 4. Despite advances in surgical techniques and radiotherapy in the last 20 years, even with the most modern techniques there continues to be a significant incidence of tumor recurrence, up to 30% or >50% in intermediate and high risk groups, respectively, highlighting the need for improved therapeutic approaches 5–8.
The use of an adjuvant tumor vaccine to combat residual tumor cells after debulking by surgery or radiation has the potential to decrease recurrence without adding toxicity. This approach could prolong disease free survival and improve quality of life by avoiding the need for castration and other subsequent treatments, all of which are essentially palliative and have significant toxicities and costs. This may be particularly impactful for patients in developing countries who may not have access to new agents for castrate-resistant prostate cancer.
Gene-Mediated Cytotoxic Immunotherapy (GMCI) is an approach that generates a tumor-specific vaccine effect through intra-tumoral delivery of an adenoviral vector containing the Herpes virus thymidine-kinase gene (AdV-tk) followed by systemic anti-herpetic prodrug (e.g. ganciclovir or valacyclovir) combined with standard debulking therapies, such as surgery and radiation 9. Initial local cytotoxicity is mediated by the nucleoside analog generated through phosphorylation of the prodrug. Cell death via necrosis and apoptosis, viral factors that stimulate the innate immune system, and the acute inflammation from the debulking therapy, attract and activate antigen presenting cells that incorporate and present tumor associated antigens (TAAs) to T cells. The HSV-TK protein, in addition to its enzymatic activity, also functions as a super-antigen like molecule potently stimulating T cell proliferation and IL-2 production9. This immunostimulatory milieu generates a systemic anti-tumor immune response to the released autologous TAAs. Preclinical studies have demonstrated the immune function of AdV-tk treatment. Treatment of local tumors with GMCI led to protection against metastases and tumor re-challenge in mouse syngeneic models 10–12. However, these distant effects were present in immunocompetent but not immunodeficient mice 12–14. Furthermore, tumor growth inhibition could be adoptively transferred with splenocytes from animals bearing tumors treated with AdV-tk plus prodrug but not from controls treated with AdV-tk plus saline 15. These studies demonstrated the immune nature of the response and showed that tumor cell death, and its subsequent TAA release, was required to induce tumor specific responses. Thus GMCI could lead to immune protection against recurrence from minimal residual disease after tumor debulking.
Although as an immunotherapy, GMCI does not require transduction of every cancer cell, it does require transduction of sufficient tumor cells to cause enough cytotoxicity for effective release of TAAs for tumor specific immune stimulation. Prostate tumors may be diffuse in the gland and may have necrotic foci. Thus, to assure vector instillation was not lost in a necrotic focus or missed tumor cell targeting, it was desirable to maximize vector distribution within the whole gland. However, evaluation of delivery methods to optimize vector distribution in prostate tumor tissue is not feasible in preclinical models. Yet, it is difficult to analyze multiple delivery volumes and site distribution in patients. It is also difficult to extrapolate the distribution of a virion, with its cellular interaction properties, using synthetic particles and expression patterns take days to develop. In the present study, ex-vivo vector distribution in freshly removed organs was evaluated using fluorescently labeled vectors to optimize delivery and in-vivo distribution from the chosen method was confirmed in the clinical study.
The current study was the first gene transfer study initiated and conducted in Latin America. The GMCI approach has demonstrated safety with potential efficacy in Phase 1 and Phase 2 clinical trials in multiple tumor types. This study further evaluates the acute and long-term safety of AdV-tk and analyzes vector distribution after multiple intra-prostatic injections. Conducting this study at the university hospital in Monterrey allowed evaluation in a setting where patients often present with high-risk characteristics and had poor long-term prognosis with standard of care. Long-term follow-up has allowed the observation of the impact of GMCI clinically and biochemically.
Materials and methods
Vector
The AdV-tk vector used here is an E1/E3 deleted replication deficient vector with the RSV promoter driving the HSV thymidine kinase gene substituted in the E1 region based on the serotype 5 backbone. Its development and production have been previously described 16,17. The clinical grade vector was produced in the Baylor College of Medicine Gene Vector Laboratory, in accordance with good manufacturing practice (21 CFR 210 and 211). The vector was transported to the clinical site with prior authorization of the General Directory of the Health Regulation Service of the Mexican Secretariat of Health (Oficio 00219. July 8th, 1999) and U.S. FDA.
Ex vivo distribution analysis
For visualization of vector distribution after multiple site and volume combinations, vector virions were conjugated to isothiocyanate, a fluorescent marker. Virion injections took place in the operating room into resected prostates immediately following radical prostatectomy. A 20 gauge 20 cm needle, similar to that used in the clinical protocols, was used to inject the vector. The volumes evaluated were 1ml and 2 mls into 1, 2 or 4 sites in the prostate. The prostates were positioned with Denovilliers’ fascia placed anteriorly so that injections would enter the posterior prostate or peripheral zone first, as would occur during transrectal ultrasound guided injections. Following injection, the specimens were placed in formalin fixative for approximately 24 hours before making multiple 3mm transverse cuts of the prostate. Each section was then evaluated using a fluorescent stereoscope to visualize the distribution of the adenoviral virions throughout the prostate.
Clinical Protocol
The clinical protocol was approved by the Research and Ethics Committee at the University Hospital of the Universidad Autonoma de Nuevo Leon (BI99-24), the Ethics Committee of the Secretariat of Health of the State of Nuevo Leon and by the General Directory of Health Services of the Mexican Secretariat of Health (File 204/002397, July 23rd,1999). All patients were thoroughly informed about the experimental protocol and counseled regarding the optimal treatment available to them prior to signing an informed consent form.
Eligibility Criteria
Patients were eligible for participation in this study if they had a pathological diagnosis of adenocarcinoma of the prostate and intended to undergo surgical resection. Diagnosis was determined by fine needle biopsy and, if indicated, patients underwent pelvic and abdominal computerized tomography scans to rule out advanced disease. Prognostic risk profiles were classified based on the National Comprehensive Cancer Network (NCCN) guidelines: low risk: T1-T2a, Gleason score of 2–6, and PSA < 10 ng/ml; intermediate risk: T2b-T2c, and/or Gleason score of 7 and/or PSA 10–20 ng/ml and no high risk features; high risk: T3, and/or Gleason score 8–10 and/or PSA > 20 ng/ml 18. To be eligible, patients were required to have a Karnofsky performance score (KPS) of ≥70, be ≤70 years old and have adequate baseline organ and coagulation function with serum creatinine ≤1.5 mg/dl, absolute platelet count ≥100, 000/μl, absolute neutrophils ≥1000/cm3, hemoglobin ≥10 mg/dl, AST and ALT <4X normal limit, total bilirubin ≤2.5 mg/dl, and PT and PTT within normal limits. Exclusion criteria included concomitant infection (including HIV), uncontrolled systemic disease, and previous treatment for prostate cancer.
Course of treatment
Prior to the procedure, the patients were prepared with oral ciprofloxacin (500 mg/12 hours, 24 hours in advance) and a phosphate enema on the morning of the injection. Blood and urine samples were taken to establish baseline values. Each patient received a four quadrant intra-prostatic injection of 2 ml total (0.5 ml per quadrant) of AdV-tk for a total dose of 1×1011 vp. Instillations were guided by transrectal ultrasonography. Based on availability of prodrug, seven patients received intravenous infusions of gancyclovir (Cimevene®, Roche-Syntex, Mexico City, Mexico) at 5 mg/kg weight every 12 hours for 14 days and three patients received 2 grams of orally administered valacyclovir (Valtrex®, Glaxo Wellcome, Research Triangle Park, NC) three times a day for 14 days. Patients underwent radical prostatectomy with an average interval of 9 weeks after injection and received subsequent clinical follow-up.
Patient follow up
Serum PSA levels were analyzed at baseline and 3, 8, 15 and 30 days after AdV-tk injection and then every month. After surgery, PSA was measured at months 1, 3, 6 and every 6 months thereafter or as clinically indicated. Toxicities were graded based on the Cancer Therapy Evaluation Program (CTEP) Common Toxicity Criteria (CTC), version 2.0.
In-vivo Distribution Analysis
In-vivo vector distribution was analyzed by PCR from prostatectomy tissue of the first four patients injected. Fresh prostate specimens were sliced in 4–6 sections. Each slice was divided in quadrants and a peri-urethral zone. Punch biopsies were taken from each of the five zones for DNA isolation. The rest of the tissue was submitted for histologic analysis. PCR primers detected a segment containing the RSV promoter and HSV-tk gene. The sensitivity limit was 100 genome copies in 150ug of genomic DNA.
Histologic Analysis
Tumor specimens were fixed in 10% neutral buffered formalin. Final diagnosis was confirmed including Gleason score, evaluation of angio-lymphatic and perineural invasion, presence of tumor at surgical borders and invasion of tumor to adjacent structures. The pathology of the prostate tissue distal to the tumor was also evaluated. Specimens were also submitted to immunohistochemical analysis with monoclonal antibodies directed against CD20, CD3, CD4, CD8 and TIA-1 (DAKO®). Inflammatory cells were evaluated using a semiquantitative scale (+: scarce isolated lymphocytes in the stroma, surrounding neoplastic cells; ++: stromal lymphocytes and other mononuclear cells in small groups, scarce leucocytes including polymorphonuclear cells involving neoplastic glands; +++: intense inflammatory reaction in both the stroma and the neoplastic component).
Biostatistical Analysis
The biostatistical analysis for this phase I/II study was descriptive. Day 0 is defined as the date of the radical prostatectomy.
Results
Distribution in ex-vivo injected prostates
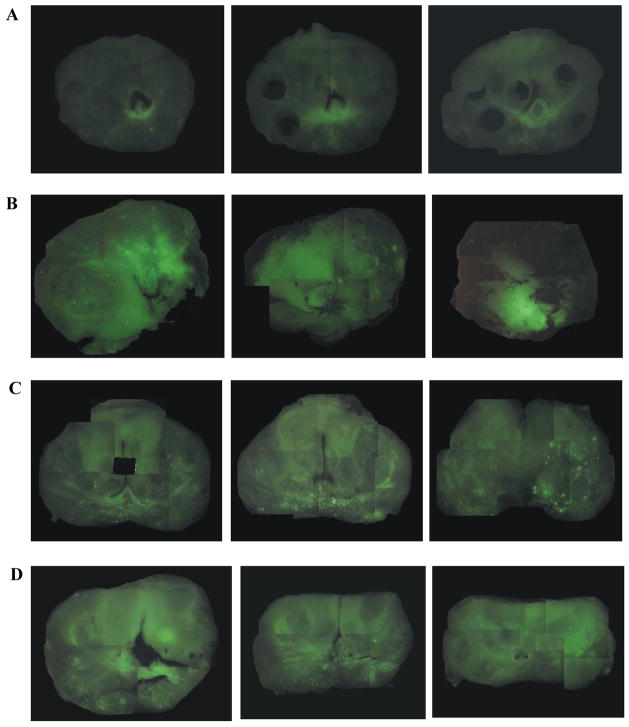
Injection at a single site within the prostate resulted in a localized distribution of the vector with either 1ml (Fig. 1, row B), or 2 ml (data not shown) with some variability in each case. Delivery of two injections of 1 ml each, one to each lobe of the prostate, increased the area of distribution within the prostate, with some variability within each lobe (Fig. 1, row C). Physical distribution by delivery of four 0.5 ml injections, one apical and one basal in each lobe, resulted in a consistent broader glandular distribution (Fig. 1, row D). The four site approach was chosen for clinical evaluation.
Figure 1. Fluorescently labeled adenoviral vector distribution analysis.
Adenoviral vector conjugated to isothiocyanate, a fluorescent marker, was injected into freshly resected prostates as described. Whole mount sections were visually analyzed using a fluorescent stereoscope. Representative examples from each of four conditions are shown above with three sections of each from apex to base shown from left to right. A) Control prostate without vector injection; note slight background fluorescence. B) Prostate after a single 1 ml injection into a lateral lobe; note localized fluorescence. C) Two one ml vector injections, one on each side of the prostate showing typical punctate distribution. D) Four injections, two on each side of the prostate, with 0.5 mL each. Fluorescence was typically diffuse throughout the organ. The latter was the method chosen for subsequent clinical studies.
Clinical trial patients
Between October 1999 and November 2001, 10 patients with newly diagnosed prostate cancer were enrolled. Seven patients were diagnosed with high-risk, one with intermediate and two with low-risk disease (Table 1). The median age was 56.5 years, Gleason scores ranged from 5–8 and baseline PSA levels ranged from 2.8–22.7 ng/ml. Surgery was performed in 9 of the 10 patients within 7.3–15.7 weeks (average interval time of 9 weeks) after vector injection. Eight patients had prostate adenocarcinoma confined to the gland and one patient had seminal vesicle involvement. Six patients had positive surgical borders (Table 2). Patient A5 was a mid-treatment dropout who refused surgery or further follow up for personal reasons.
Table 1.
Baseline Patient Characteristics
| Patient | Age (years) | Stage | PSA (ng/ml) | Prostate Volume (grams) | Gleason Score | Risk Group^ | Interval gene transfer to RP (weeks) |
|---|---|---|---|---|---|---|---|
| A1 | 46 | pT2a | 5.8 | 22 | 5(3+2) | Low | 7.4 |
| A2 | 59 | pT3a | 15.6 | 14.5 | 7(3+4) | High | 10.4 |
| A3 | 66 | pT3a | 2.81 | 21 | 7(3+4) | High | 15.7 |
| A4 | 50 | pT2a | 8.85 | 80 | 5(2+3) | Low | 14.7 |
| A5* | 62 | ------- | 22.7 | ------- | n/a | High | ------- |
| A6 | 65 | pT3b | 11.8 | 48 | 8(4+4) | High | 9.4 |
| A7 | 54 | pT3a | 9.6 | 23 | 7(4+3) | High | 8.1 |
| A8 | 53 | pT2c | 4.53 | 12.5 | 7(3+4) | Intermediate | 8.3 |
| A9 | 61 | pT3a | 9 | 20 | 7(3+4) | High | 8.3 |
| A10 | 49 | pT3a | 3.4 | 23 | 7(3+4) | High | 7.3 |
Dropped out of study due to declining surgery
Based on National Comprehensive Cancer Network (NCCN) Guidelines
Table 2.
Histological Analysis of Resected Prostates
| Patient | Gleason | Vascular Invasion | Perineural Invasion | Surgical Borders | Invasion of adjacent structures | Inflammatory Response |
|---|---|---|---|---|---|---|
| A1 | 5 | − | − | − | No | − |
| A2 | 7 | + | + | + | No | +++ |
| A3 | 7 | + | + | + | No | ++ |
| A4 | 5 | − | − | − | No | ++ |
| A6 | 8 | + | + | + | Seminal Vesicle | + |
| A7 | 7 | + | + | + | No | +++ |
| A8 | 7 | − | + | − | No | + |
| A9 | 7 | + | + | + | No | +++ |
| A10 | 7 | − | + | + | No | ++ |
scarce isolated lymphocytes in the stroma, surrounding neoplastic cells;
stromal lymphocytes and other mononuclear cells in small groups, scarce leucocytes including polymorphonuclear cells involving neoplastic glands;
intense inflammatory reaction in both the stroma and the neoplastic component
Distribution in prostates injected in-vivo
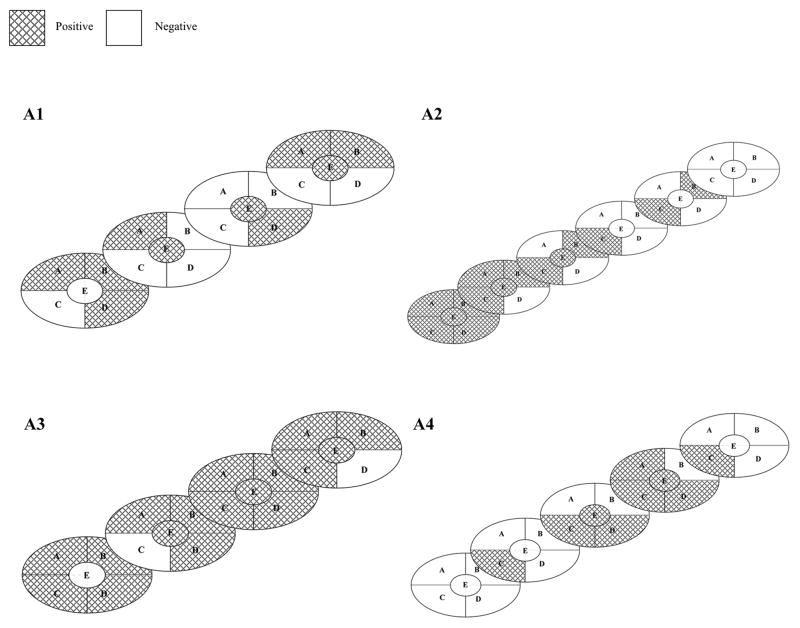
AdV-tk distribution was analyzed in resected prostates from four patients who had surgery 7.4 to 15.7 weeks after injection. Vector sequences were detected dispersed throughout the prostate in forty to 85% of zones analyzed: 10/20, 14/28, 17/20 and 9/23 for patients A1, A2, A3 and A4, respectively (Figure 2).
Figure 2. Intra-prostatic vector DNA distribution.
Freshly resected prostates were sliced from anterior to posterior. Each slice was divided into four quadrants (A, B, C, D) and a center peri-urethral zone (E). Punch biopsies were taken from each of the five zones for DNA isolation and analyzed by PCR. Graphic representation of sliced prostates: areas positive for vector DNA are depicted by cross-hatch and negative areas are blank.
Histologic Analysis
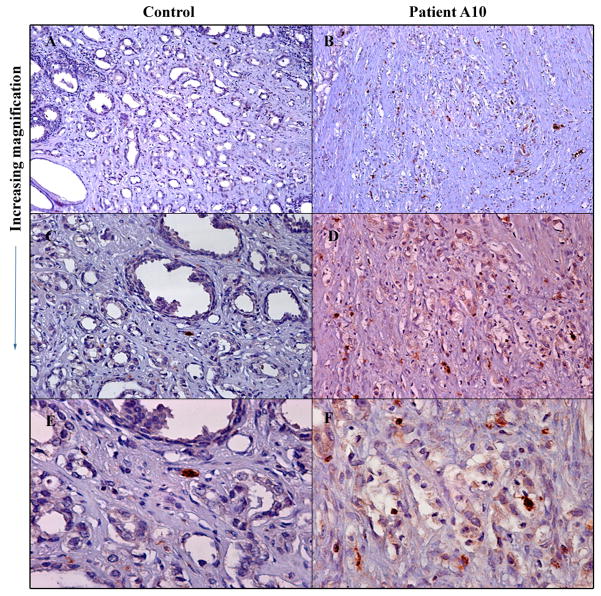
Nine prostate specimens were analyzed for necrosis, apoptosis and inflammatory response was (Table 2). Characterization of inflammatory infiltrate revealed a predominance of T cells compared to B cells (ratio 4:1) in neoplastic areas with a CD4:CD8 ratio of 1:4. CD4 cells were predominantly found in the stroma. TIA-1 (T-cell intracytoplasmic antigen) is a granule-associated RNA binding protein expressed in cells with cytolytic potential, including activated CD8+ T cells and NK cells 19. As shown in Figure 3, AdV-tk treated prostate tumors had an increased infiltration of TIA-1+ cells compared to untreated control.
Figure 3. Immune cytolytic cell infiltration in treated tumor compared to untreated control tumor.
Resected prostate tumors were processed and immunohistochemical analysis performed with antibody to human TIA-1 followed by immunoperoxidase staining using avidin-biotin complex. A and B, 10X; C and D, 20X and E and F, 40X.
Safety
No dose limiting toxicities occurred. Adverse events deemed possibly related to AdV-tk + prodrug are listed in Table 3. The most common event was grade-1 flu-like symptoms observed in 3 patients in the first 48 hours post-injection. Other events were laboratory abnormalities that resolved without clinical consequence or intervention.
Table 3.
Possibly Related Adverse Events
| Patient | Adverse Event | Grade (CTC) | Outcome |
|---|---|---|---|
| A2, A6 and A9 | Flu-like symptoms | 1 | Resolved |
| A9 | Leucopenia | 1 | Resolved |
| A9 | Lymphopenia | 3 | Resolved |
| A2 | Increased AST | 1 | Resolved |
| A8 | Increased ALT | 1 | Resolved |
| A2 | Increased total bilirubin | 2 | Resolved |
Unrelated events included two patients that developed urinary tract infections 3–4 days after the injection, likely due to urinary catheter usage. These were successfully treated with antibiotics. Another patient developed a ventricular arrhythmia 1 week after injection, deemed most likely related to uncontrolled chronic hypertension. It resolved without evidence of ischemia or other abnormalities.
After a median follow-up of 11.3 years, no late toxicities have been reported. Six patients were still alive at last follow up 10–13 years after surgery (Table 4). Three patients died of unrelated causes without additional treatment for their prostate cancers 6–12 years after surgery.
Table 4.
Patient Follow Up and Overall Survival
| Patient | Late toxicities | Prostate Cancer Status | Survival Status | Overall Survival (Years) |
|---|---|---|---|---|
| A1 | None | NED | Alive | 12.6 |
| A2 | None | Slow PSA rise, metastatic workup neg | Died of AMI | 12.3 |
| A3 | None | NED | Died of esophageal cancer | 9.9 |
| A4 | None | NED | Alive | 13.2 |
| A6 | None | NED | Died of AMI | 6.7 |
| A7 | None | Slow PSA rise, metastatic workup neg | Alive | 12.4 |
| A8 | None | NED | Alive | 10.7 |
| A9 | None | Slow PSA rise on ADT, metastatic workup neg | Alive | 11.1 |
| A10 | None | NED | Alive | 10.3 |
NED- no evidence of disease, ADT- androgen deprivation therapy, AMI- acute myocardial infarction
PSA and Clinical Response
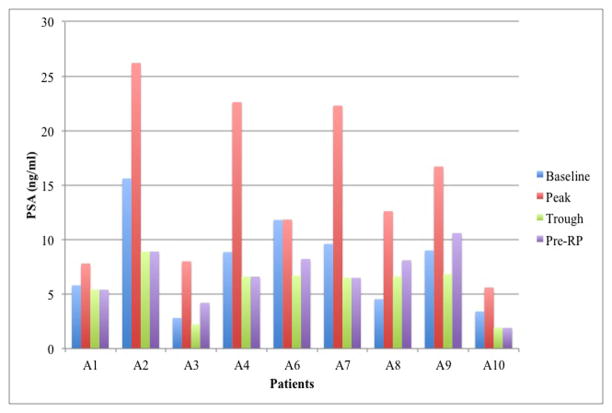
After AdV-tk injection, serum PSA levels rose to a peak during week 1–2 and then fell prior to surgery in 8 of 9 patients. PSA levels for patient A6 did not rise after injection but still decreased below baseline prior to surgery (Figure 4A). These PSA changes indicate cytotoxic activity of AdV-tk + prodrug.
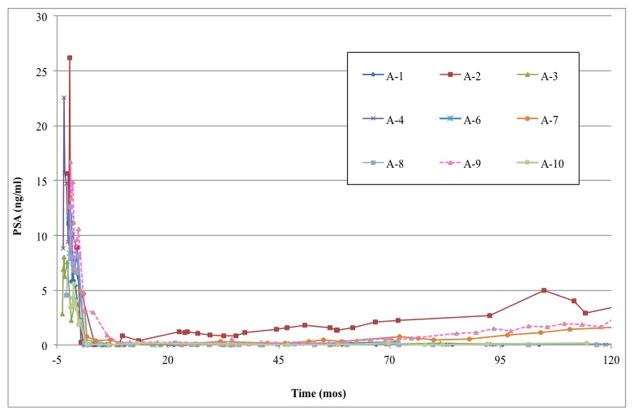
Figure 4. Serum PSA changes after AdV-tk injection before radical prostatectomy (A) and continuing on through long-term follow up (B).
PSA was measured at baseline and 3, 8, 15 and 30 days after AdV-tk injection and then every month until surgery. After surgery, PSA was measured at months 1, 3, 6 and every 6 months thereafter or as clinically indicated.
A post-RP PSA pattern of rapid decline to zero (rapid zero pattern) occurred in 7 patients within 6 months of surgery, even though 4 of these patients had positive surgical borders and one had seminal vesicle involvement (Figure 3B). A different post-RP pattern (slow decline pattern) was observed in patient A7, who had a drop in PSA to 0.78 ng/ml within 2 months after surgery followed by a gradual decrease to 0.1 by 10 months. Patient A9 was considered to have PSA failure immediately after surgery due to a persistent PSA level of 3 ng/ml at 3 months and was started on androgen deprivation therapy (ADT) with bicalutamide.
With long-term follow up, 6 of the 7 patients with the rapid zero pattern continued to have very low PSA and no other evidence of disease ≥10 years after RP or, in one case, at the time of unrelated death from myocardial infarction 6.7 years after RP (Figure 4B and Table 4). The other patient with the rapid zero pattern (patient A2) and patient A7, with the initial slow decline pattern, had a slow rise over 10 years without exceeding 5 ng/ml without clinical evidence of disease and with negative imaging for metastases. Patient A9 had a similar pattern of slow PSA rise on bicalutamide without metastases > 10 years after RP.
Discussion
Conceptually, the immune system is most likely to be effective against minimal tumor burdens rather than large, late stage tumors. The latter, by definition, have already overcome the immune system and often have evolved systems that thwart immunity. Therefore, immunotherapy is most likely to be effective when used against minimal residual disease rather than as primary therapy for bulky or late stage disease. Local delivery of a cytotoxic agent with immunostimulatory properties can provide the geographic and temporal co-localization of tumor associated antigens and immunostimulation to generate a tumor specific vaccine that will inhibit tumor cell proliferation.
Tumor cell killing by AdV-tk + prodrug is required to release the TAA and stimulate antitumor immunity, as evidenced in animal models 15. Therefore, although transduction of every tumor cell is not required, a delivery method that improves vector distribution throughout the prostate gland maximizes the probability for adequate delivery to tumor cells in the prostate gland. This is important to assure release of TAA and create the immunostimulatory milieu necessary for the in situ vaccine effect 9. To optimize delivery to tumor cells throughout the prostate in a systematic way, different volumes of vector and sites of delivery were evaluated. Ex vivo analysis with fluorescein-labeled vector allowed easy analysis of multiple approaches and subsequent in vivo delivery confirmed that this method provided good distribution throughout the prostate. These results are applicable to intra-prostatic delivery of adenoviral vectors in general with the same viral capsid since the biological determinants of transduction, distribution and persistence in vivo are based on the protein components of the capsid independent of the genomic content of the vector.
The 4-site intra-prostatic delivery of AdV-tk lead to broad distribution of vector throughout the prostate and resulted in necrosis and apoptosis as well as immune cell infiltration in the tumor. Additional evidence of tumor cell cytotoxicity was the transient rise in PSA observed after prodrug administration. This instillation method is practical for use as an outpatient procedure and well tolerated. This is the method being used for an ongoing phase 3 randomized trial of AdV-tk + valacyclovir in combination with radiation for localized prostate cancer (ProstAtak trial).
The study presented here expanded the safety data for AdV-tk + prodrug and was the first clinical gene therapy study initiated and conducted in Latin America. The AdV-tk vector dose (1×1011 vp) was based on previous dose escalation studies in prostate cancer 17,27. Overall, the approach was well tolerated with the most common adverse event being mild flu-like symptoms during the first 48 hours after injection likely due to the inflammatory response to the AdV-tk vector. The only grade 3 adverse event was lymphopenia that occurred during ganciclovir administration in one patient. This abnormality resolved without treatment or complications. No late toxicities occurred after more than 10 years of surveillance, demonstrating long-term safety.
Continuous follow-up of over 10 years has allowed the evaluation of long-term effects and the clinical outcome of patients. Despite high-risk features in 6 patients including positive surgical margins and seminal vesicle involvement, none of the patients developed metastases or death from prostate cancer. Although there is not a consensus on the definition of biochemical failure following prostatectomy, most studies use a value of 0.2 ng/ml or 0.4 ng/ml 5,28,29. In a retrospective study of outcomes of 1997 men with clinically localized prostate cancer undergoing radical prostatectomy at Johns Hopkins University, PSA failure occurred in 15% of patients of which metastases developed in 34% without treatment at the time of PSA failure 5. Patients with locally advanced disease (capsular perforation, positive margins and/or seminal vesicle involvement) have a higher risk of PSA recurrence and metastases. Two randomized studies (EORTC 22911 and SWOG 8794) evaluated adjuvant post-operative radiation in these patients compared to delaying additional treatment until PSA recurrence 28,29. Both studies found a significant improvement in recurrence free survival with immediate adjuvant radiation although with increased late adverse events. In the EORTC study, PSA dropped to ≤0.2 ng/ml within 4 months after RP in 70% of patients before radiation and recurrence occurred in 52% of the delayed treatment group versus 23% of the adjuvant group 28. In the SWOG study, PSA relapse was 64% in the delayed group and 35% in the adjuvant group 29. In our study, within 4 months after RP, 7/9 patients achieved a PSA ≤0.2 ng/ml, 1 achieved PSA <0.4 ng/ml and 1 patient was started on bicalutamide for PSA failure. Subsequently, 2 patients developed PSA > 0.4 ng/ml but were not treated for recurrence and had PSADT of 28 and 35 months. PSADT of >10 months has been associated with decreased risk of metastases and prostate cancer death 5,30. In our study, no patient has developed metastases or died of prostate cancer despite 6 of the 9 patients being locally advanced. These results are better than expected for this patient population especially without receiving additional treatment.
Preclinical studies demonstrated that the local and systemic effects of AdV-tk are potentiated when used in combination with standard debulking therapies such as surgery, radiation and some chemotherapeutic agents 20–23. In murine prostate and mammary models, AdV-tk delivered to the tumor bed following surgical resection delayed local recurrence and inhibited lung metastases 21. CD8 T cells and NK cells have been shown to be important for the systemic effect of AdV-tk + prodrug 15,24. In a lung tumor model, potentiation of the systemic effect by subsequent cytoreductive surgery was shown to be CD8 T-cell dependent using a Winn assay 25. Mechanistically, this was shown to be due, at least in part, to a reduction in systemic myeloid derived suppressor cells (MDSCs) after surgery 25. In the current study, prostates from patients treated with AdV-tk + prodrug had increased infiltration of TIA+ cytotoxic effector cells compared to control prostates. Abundance of TIA-1+ cells has been shown to be a good prognostic feature in some cancer types 19. Another study evaluating AdV-tk + prodrug prior to prostatectomy found increased infiltration of CD8+ cells compared to controls and increased circulating DR+CD8 T cells compared to baseline before AdV-tk + prodrug 26. These data strongly suggest that adjuvant use of AdV-tk + prodrug stimulates an anti-tumor immune response and may significantly prolong or inhibit the recurrence of a clinically meaningful tumor burden in prostate cancer patients.
Intra-prostatic injections of AdV-tk, as adjuvant treatment for localized prostate cancer, has been demonstrated to be safe and feasible in an outpatient setting. More than 10 years of follow-up in the current study has allowed for the observation of the long-term safety.
Using antitumor immunotherapy with surgery to increase the host’s adaptive immune system to minimize residual cancer cells after standard of care debulking therapy, may increase the patient’s metastasis free survival without added toxicity by generating a long-standing anti-tumor memory. This offers additional protection for patients that have a high risk of recurrence. Slow decline and slow rising PSA patterns seen in this study suggest that anti-tumor immunity may have been keeping residual cancer cells in check in these high risk patients, preventing clinical progression. The use of GMCI with surgery and radiation to target minimal residual disease has demonstrated great potential by acting synergistically with standard of care 20,21,31. Efficacy of the approach in combination with surgery will require a definitive study such as the ProstAtak trial currently being conducted in combination with radiation therapy. The ability to easily deliver AdV-tk to the prostate in a simple, outpatient procedure to generate a patient-specific in-situ vaccine is a unique paradigm that can facilitate bringing immunotherapy to patients throughout the world.
Acknowledgments
The authors would like to acknowledge and thank the patients that volunteer to give of their time and effort for these clinical trials in the hope of improving medical care for future generations. We would also like to acknowledge Dr. Jesús Zacarías Villarreal Pérez, Minister of Health for the State of Nuevo Leon, who placed his support and effort to acquire the stringent regulatory approvals to import the vector and conduct the first gene therapy trial in the country. Supported in part by Grant No. R44CA124032 from the National Cancer Institute.
Footnotes
Conflict of Interest: The authors identified as members of Advantagene, Inc. are employed by the company, which holds an interest in the AdV-tk technology. The other authors received no compensation and have no financial interest or benefit from the research presented here.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, et al. Cancer Incidence and Mortality Worldwide. GLOBOCAN 2008 v2.0. 2010 at < http://globocan.iarc.fr>.
- 3.Gomez-Guerra LS, et al. Population based prostate cancer screening in north Mexico reveals a high prevalence of aggressive tumors in detected cases. BMC Cancer. 2009;9:91. doi: 10.1186/1471-2407-9-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walsh PC, DeWeese TL, Eisenberger MA. Clinical practice. Localized prostate cancer. N Engl J Med. 2007;357:2696–705. doi: 10.1056/NEJMcp0706784. [DOI] [PubMed] [Google Scholar]
- 5.Pound CR, et al. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281:1591–7. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 6.Simmons MN, Stephenson AJ, Klein EA. Natural history of biochemical recurrence after radical prostatectomy: risk assessment for secondary therapy. Eur Urol. 2007;51:1175–84. doi: 10.1016/j.eururo.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 7.Zelefsky MJ, et al. Dose escalation for prostate cancer radiotherapy: predictors of long-term biochemical tumor control and distant metastases-free survival outcomes. Eur Urol. 2011;60:1133–9. doi: 10.1016/j.eururo.2011.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bolla M, Verry C, Long JA. High-risk prostate cancer: combination of high-dose, high-precision radiotherapy and androgen deprivation therapy. Curr Opin Urol. 2013;23:349–54. doi: 10.1097/MOU.0b013e328361ebfd. [DOI] [PubMed] [Google Scholar]
- 9.Aguilar LK, Guzik BW, Aguilar-Cordova E. Cytotoxic immunotherapy strategies for cancer: mechanisms and clinical development. J Cell Biochem. 2011;112:1969–77. doi: 10.1002/jcb.23126. [DOI] [PubMed] [Google Scholar]
- 10.Hall SJ, Mutchnik SE, Chen SH, Woo SL, Thompson TC. Adenovirus-mediated herpes simplex virus thymidine kinase gene and ganciclovir therapy leads to systemic activity against spontaneous and induced metastasis in an orthotopic mouse model of prostate cancer. Int J Cancer. 1997;70:183–7. doi: 10.1002/(sici)1097-0215(19970117)70:2<183::aid-ijc8>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 11.Perez-Cruet MJ, et al. Adenovirus-mediated gene therapy of experimental gliomas. J Neurosci Res. 1994;39:506–11. doi: 10.1002/jnr.490390417. [DOI] [PubMed] [Google Scholar]
- 12.Vile RG, Nelson JA, Castleden S, Chong H, Hart IR. Systemic gene therapy of murine melanoma using tissue specific expression of the HSVtk gene involves an immune component. Cancer Res. 1994;54:6228–34. [PubMed] [Google Scholar]
- 13.Gagandeep S, et al. Prodrug-activated gene therapy: involvement of an immunological component in the “bystander effect”. Cancer Gene Ther. 1996;3:83–8. [PubMed] [Google Scholar]
- 14.Kuriyama S, et al. Cancer gene therapy with HSV-tk/GCV system depends on T-cell-mediated immune responses and causes apoptotic death of tumor cells in vivo. Int J Cancer. 1999;83:374–80. doi: 10.1002/(sici)1097-0215(19991029)83:3<374::aid-ijc13>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 15.Agard C, et al. Immune-dependent distant bystander effect after adenovirus-mediated suicide gene transfer in a rat model of liver colorectal metastasis. Cancer Gene Ther. 2001;8:128–36. doi: 10.1038/sj.cgt.7700281. [DOI] [PubMed] [Google Scholar]
- 16.Chen SH, Shine HD, Goodman JC, Grossman RG, Woo SL. Gene therapy for brain tumors: regression of experimental gliomas by adenovirus-mediated gene transfer in vivo. Proc Natl Acad Sci U S A. 1994;91:3054–7. doi: 10.1073/pnas.91.8.3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herman JR, et al. In situ gene therapy for adenocarcinoma of the prostate: a phase I clinical trial. Hum Gene Ther. 1999;10:1239–49. doi: 10.1089/10430349950018229. [DOI] [PubMed] [Google Scholar]
- 18.Mohler JL, et al. Prostate cancer, Version 3.2012: featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2012;10:1081–7. doi: 10.6004/jnccn.2012.0114. [DOI] [PubMed] [Google Scholar]
- 19.Zlobec I, et al. TIA-1 cytotoxic granule-associated RNA binding protein improves the prognostic performance of CD8 in mismatch repair-proficient colorectal cancer. PLoS One. 2010;5:e14282. doi: 10.1371/journal.pone.0014282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chhikara M, et al. Enhanced therapeutic effect of HSV-tk+GCV gene therapy and ionizing radiation for prostate cancer. Mol Ther. 2001;3:536–42. doi: 10.1006/mthe.2001.0298. [DOI] [PubMed] [Google Scholar]
- 21.Sukin SW, et al. In vivo surgical resection plus adjuvant gene therapy in the treatment of mammary and prostate cancer. Mol Ther. 2001;3:500–6. doi: 10.1006/mthe.2001.0285. [DOI] [PubMed] [Google Scholar]
- 22.Nestler U, et al. The combination of adenoviral HSV TK gene therapy and radiation is effective in athymic mouse glioblastoma xenografts without increasing toxic side effects. J Neurooncol. 2004;67:177–88. doi: 10.1023/b:neon.0000021897.53969.ca. [DOI] [PubMed] [Google Scholar]
- 23.Rainov NG, et al. Temozolomide enhances herpes simplex virus thymidine kinase/ganciclovir therapy of malignant glioma. Cancer Gene Ther. 2001;8:662–8. doi: 10.1038/sj.cgt.7700355. [DOI] [PubMed] [Google Scholar]
- 24.Hall SJ, Sanford MA, Atkinson G, Chen SH. Induction of potent antitumor natural killer cell activity by herpes simplex virus-thymidine kinase and ganciclovir therapy in an orthotopic mouse model of prostate cancer. Cancer Res. 1998;58:3221–5. [PubMed] [Google Scholar]
- 25.Predina JD, et al. Cytoreduction surgery reduces systemic myeloid suppressor cell populations and restores intratumoral immunotherapy effectiveness. J Hematol Oncol. 2012;5:34. doi: 10.1186/1756-8722-5-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ayala G, et al. Biological response determinants in HSV-tk + ganciclovir gene therapy for prostate cancer. Mol Ther. 2006;13:716–28. doi: 10.1016/j.ymthe.2005.11.022. [DOI] [PubMed] [Google Scholar]
- 27.Van der Linden RRM, et al. Virus specific immune responses after human neoadjuvant adenovirus-mediated suicide gene therapy for prostate cancer. Eur Urol. 2005;48:153–61. doi: 10.1016/j.eururo.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 28.Bolla M, et al. Postoperative radiotherapy after radical prostatectomy for high-risk prostate cancer: long-term results of a randomised controlled trial (EORTC trial 22911) Lancet. 2012;380:2018–27. doi: 10.1016/S0140-6736(12)61253-7. [DOI] [PubMed] [Google Scholar]
- 29.Thompson IM, et al. Adjuvant radiotherapy for pathologically advanced prostate cancer: a randomized clinical trial. JAMA. 2006;296:2329–35. doi: 10.1001/jama.296.19.2329. [DOI] [PubMed] [Google Scholar]
- 30.Freedland SJ, et al. Death in patients with recurrent prostate cancer after radical prostatectomy: prostate-specific antigen doubling time subgroups and their associated contributions to all-cause mortality. J Clin Oncol. 2007;25:1765–71. doi: 10.1200/JCO.2006.08.0572. [DOI] [PubMed] [Google Scholar]
- 31.Chiocca EA, et al. Phase IB study of gene-mediated cytotoxic immunotherapy adjuvant to up-front surgery and intensive timing radiation for malignant glioma. J Clin Oncol. 2011;29:3611–9. doi: 10.1200/JCO.2011.35.5222. [DOI] [PMC free article] [PubMed] [Google Scholar]