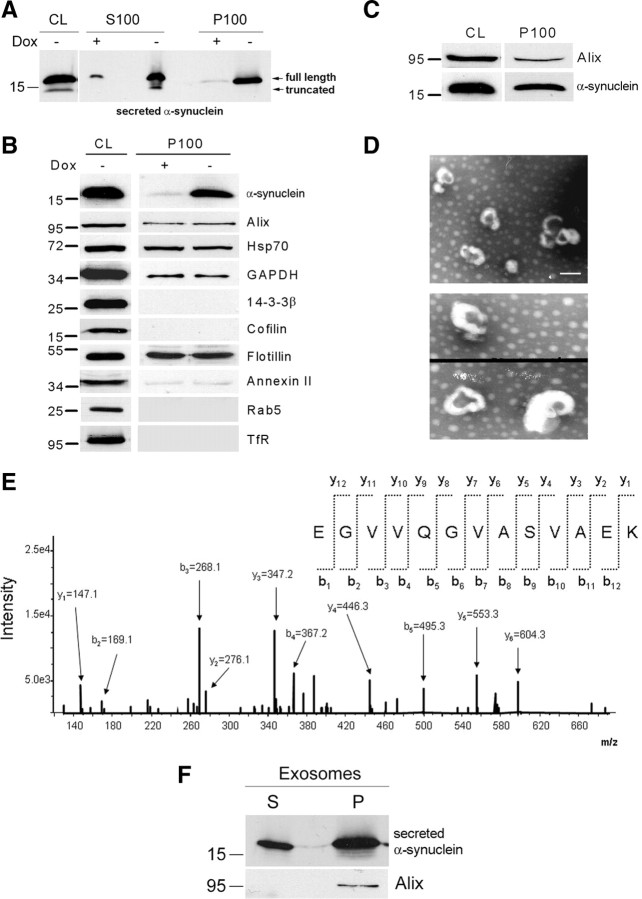
Figure 3.
α-Synuclein exocytosis is mediated by membrane vesicles that resemble exosomes. A, α-Synuclein-expressing cells and nonexpressing CTL cells were cultured in 2% FBS for 48 h. After ultracentrifugation of the CM, the membrane (P100) and the soluble (S100) fractions were analyzed for α-synuclein with the polyclonal C-20 antibody. B, P100 pellets were reconstituted in RIPA buffer and analyzed by Western immunoblotting with antibodies against the proteins indicated. TfR, Transferrin receptor. C, P100 pellet representing pure exosomes was analyzed using antibodies against α-synuclein (C-20) and Alix. D, Externalized membrane vesicles were prepared as in A. After fixation, vesicles were negatively stained with 2% uranyl acetate and observed by electron microscopy. Scale bar, 100 nm. E, Analysis of externalized membrane vesicles by mass spectrometry. The annotated product ion MS2 spectrum of the tryptic peptide EGVVQGVASVAEK traceable to α-synuclein is depicted. F, The P100 fraction containing exosomes was treated with Na2CO3. After a 50,000 × g centrifugation, the integral membrane proteins were recovered in the pellet (P), whereas non-integral and lumen proteins remained in the supernatant (S). P and S were analyzed for α-synuclein by immunoblotting with the C-20 antibody.