Figure 5.
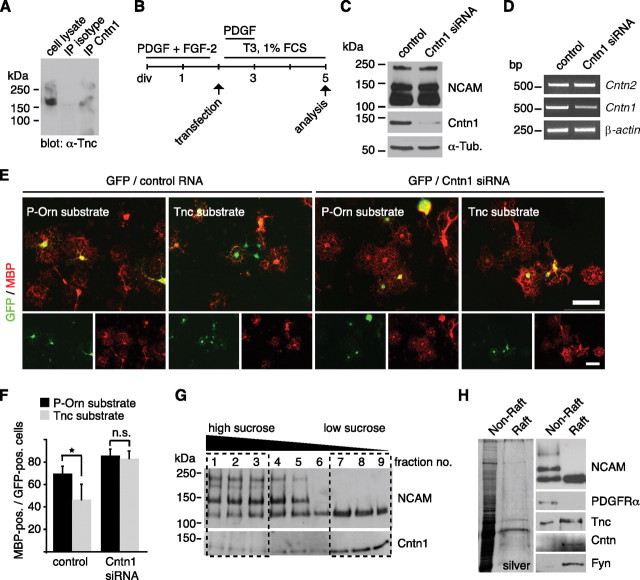
Tnc-mediated inhibition of MBP expression depends on Cntn1 and both molecules are present in lipid rafts. A, Western blotting for Tnc shows that Tnc coprecipitates when Cntn1 was immunoprecipitated from mixed glial cultures. This is not the case when a nonspecific mouse IgG was used. B, Schematic drawing of the cultivation protocol for OPC transfection with siRNA duplexes against Cntn1. “PDGF+FGF-2” refers to the OPC expansion phase, “T3 + 1%FCS” means differentiating culture conditions. C, Immunoblotting confirms successful knockdown of Cntn1 protein while NCAM is not affected. Tubulin served as loading control (α-Tub). D, PCR analysis after Cntn1 knockdown reveals a reduction of Cntn1, but not Cntn2 mRNA levels. Actin served as loading control. E, F, Photomicrographs of OPCs that were cultivated on P-Orn or Tnc substrates after cotransfection with GFP and siRNAs and immunostained for MBP. The quantification displays the proportion of transfected (GFP-positive) cells that coexpressed MBP on control P-Orn or Tnc substrates. The number of MBP-expressing GFP-positive cells is significantly reduced on Tnc after control transfection. This was abolished after Cntn1 knockdown (n = 4, *p ≤ 0.05). n.s., Not significant. Data are expressed as mean ± SD. G, Immunoblotting for NCAM and Cntn1 after sucrose density gradient ultracentrifugation. The 140 and 180 kDa forms of NCAM containing fractions 1–3 were designated to be the “nonraft” fraction, whereas fractions 7–9 were selected as “raft” fraction due to the accumulation of Cntn1 and the 120 kDa NCAM isoform. H, The silver-stained SDS gel shows that the raft fraction contains considerably less protein than the nonraft fraction. Nevertheless, immunoblotting reveals that Tnc is prominently detectable in the raft fraction, together with NCAM 120, Cntn1, and Fyn but not the PDGFRα. Scale bar, 100 μm.