Abstract
A purified, monoclonal antibody, specific for the left-handed Z-form of poly(dG-dC), was coupled covalently to Sephacryl S-1000 beads. Such an antibody column provides a convenient method to isolate and purify those plasmid DNAs that contain Z-DNA from a large excess of other DNAs, RNA, etc. From a library of Escherichia coli DNA, cloned into the vector plasmid pUC-8, several recombinant plasmids were isolated, which bind to this antibody. Thus, E. coli contains sequences, which in "natural" negatively supercoiled DNA, adopt a left-handed Z-DNA-like conformation.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnott S., Chandrasekaran R., Birdsall D. L., Leslie A. G., Ratliff R. L. Left-handed DNA helices. Nature. 1980 Feb 21;283(5749):743–745. doi: 10.1038/283743a0. [DOI] [PubMed] [Google Scholar]
- Bauer W. R. Structure and reactions of closed duplex DNA. Annu Rev Biophys Bioeng. 1978;7:287–313. doi: 10.1146/annurev.bb.07.060178.001443. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahms S., Vergne J., Brahms J. G., Di Capua E., Bucher P., Koller T. Natural DNA sequences can form left-handed helices in low salt solution under conditions of topological constraint. J Mol Biol. 1982 Dec 5;162(2):473–493. doi: 10.1016/0022-2836(82)90539-3. [DOI] [PubMed] [Google Scholar]
- Drew H., Takano T., Tanaka S., Itakura K., Dickerson R. E. High-salt d(CpGpCpG), a left-handed Z' DNA double helix. Nature. 1980 Aug 7;286(5773):567–573. doi: 10.1038/286567a0. [DOI] [PubMed] [Google Scholar]
- Goding J. W. Use of staphylococcal protein A as an immunological reagent. J Immunol Methods. 1978;20:241–253. doi: 10.1016/0022-1759(78)90259-4. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Ish-Horowicz D., Burke J. F. Rapid and efficient cosmid cloning. Nucleic Acids Res. 1981 Jul 10;9(13):2989–2998. doi: 10.1093/nar/9.13.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knippers R., Razin A., Davis R., Sinsheimer R. L. The process of infection with Bacteriophage phi-X174. XXIX. In vivo studies on the synthesis of the single-stranded DNA of progeny phi-X174 bacteriophage. J Mol Biol. 1969 Oct 28;45(2):237–263. doi: 10.1016/0022-2836(69)90103-x. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Kłysik J., Stirdivant S. M., Larson J. E., Hart P. A., Wells R. D. Left-handed DNA in restriction fragments and a recombinant plasmid. Nature. 1981 Apr 23;290(5808):672–677. doi: 10.1038/290672a0. [DOI] [PubMed] [Google Scholar]
- Lafer E. M., Möller A., Nordheim A., Stollar B. D., Rich A. Antibodies specific for left-handed Z-DNA. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3546–3550. doi: 10.1073/pnas.78.6.3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang M. C., Malfoy B., Freund A. M., Daune M., Leng M. Visualization of Z sequences in form V of pBR322 by immuno-electron microscopy. EMBO J. 1982;1(10):1149–1153. doi: 10.1002/j.1460-2075.1982.tb00005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malfoy B., Leng M. Antiserum to Z-DNA. FEBS Lett. 1981 Sep 14;132(1):45–48. doi: 10.1016/0014-5793(81)80424-3. [DOI] [PubMed] [Google Scholar]
- March S. C., Parikh I., Cuatrecasas P. A simplified method for cyanogen bromide activation of agarose for affinity chromatography. Anal Biochem. 1974 Jul;60(1):149–152. doi: 10.1016/0003-2697(74)90139-0. [DOI] [PubMed] [Google Scholar]
- Möller A., Gabriels J. E., Lafer E. M., Nordheim A., Rich A., Stollar B. D. Monoclonal antibodies recognize different parts of Z-DNA. J Biol Chem. 1982 Oct 25;257(20):12081–12085. [PubMed] [Google Scholar]
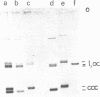
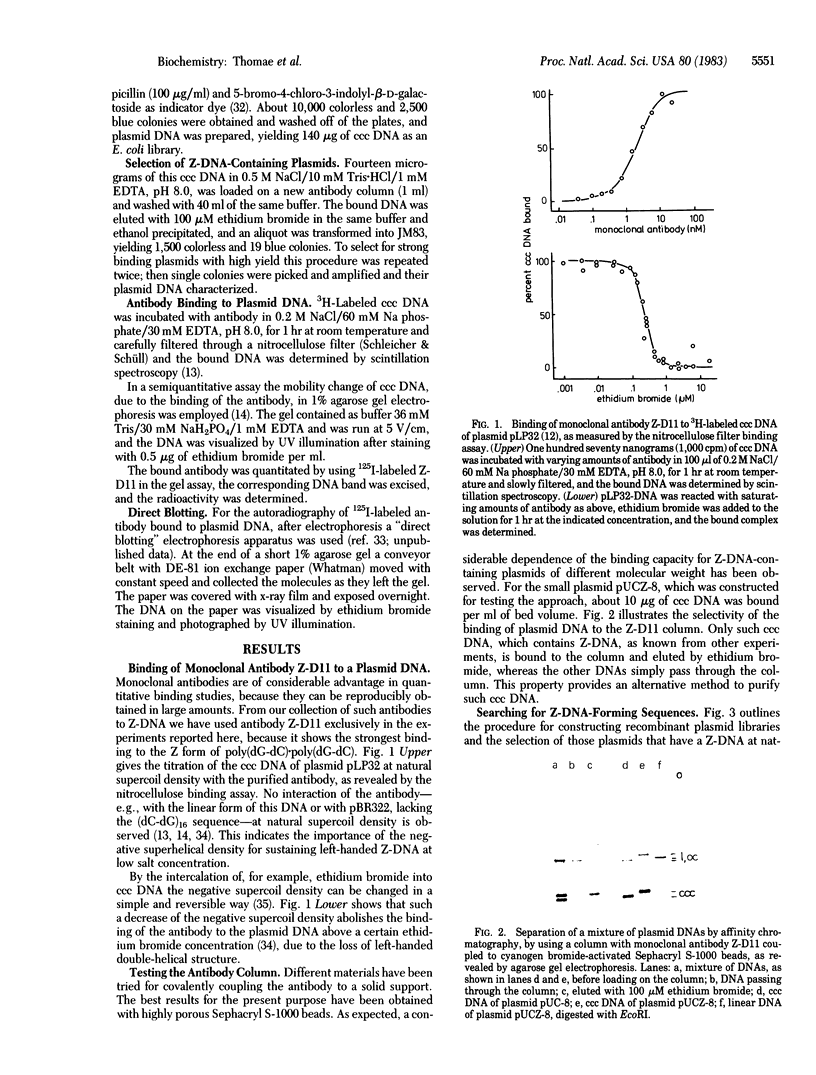
- Nordheim A., Lafer E. M., Peck L. J., Wang J. C., Stollar B. D., Rich A. Negatively supercoiled plasmids contain left-handed Z-DNA segments as detected by specific antibody binding. Cell. 1982 Dec;31(2 Pt 1):309–318. doi: 10.1016/0092-8674(82)90124-6. [DOI] [PubMed] [Google Scholar]
- Nordheim A., Pardue M. L., Lafer E. M., Möller A., Stollar B. D., Rich A. Antibodies to left-handed Z-DNA bind to interband regions of Drosophila polytene chromosomes. Nature. 1981 Dec 3;294(5840):417–422. doi: 10.1038/294417a0. [DOI] [PubMed] [Google Scholar]
- Nordheim A., Tesser P., Azorin F., Kwon Y. H., Möller A., Rich A. Isolation of Drosophila proteins that bind selectively to left-handed Z-DNA. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7729–7733. doi: 10.1073/pnas.79.24.7729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel D. J., Canuel L. L., Pohl F. M. "Alternating B-DNA" conformation for the oligo(dG-dC) duplex in high-salt solution. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2508–2511. doi: 10.1073/pnas.76.6.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck L. J., Nordheim A., Rich A., Wang J. C. Flipping of cloned d(pCpG)n.d(pCpG)n DNA sequences from right- to left-handed helical structure by salt, Co(III), or negative supercoiling. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4560–4564. doi: 10.1073/pnas.79.15.4560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl F. M., Jovin T. M. Salt-induced co-operative conformational change of a synthetic DNA: equilibrium and kinetic studies with poly (dG-dC). J Mol Biol. 1972 Jun 28;67(3):375–396. doi: 10.1016/0022-2836(72)90457-3. [DOI] [PubMed] [Google Scholar]
- Pohl F. M. Salt-induced transition between two double-helical forms of oligo (dC-dG). Cold Spring Harb Symp Quant Biol. 1983;47(Pt 1):113–117. doi: 10.1101/sqb.1983.047.01.014. [DOI] [PubMed] [Google Scholar]
- Pohl F. M., Thomae R., DiCapua E. Antibodies to Z-DNA interact with form V DNA. Nature. 1982 Dec 9;300(5892):545–546. doi: 10.1038/300545a0. [DOI] [PubMed] [Google Scholar]
- Pohl F. M., Thomae R., Karst A. Temperature dependence of the activity of DNA-modifying enzymes: endonucleases and DNA ligase. Eur J Biochem. 1982 Mar;123(1):141–152. doi: 10.1111/j.1432-1033.1982.tb06510.x. [DOI] [PubMed] [Google Scholar]
- Quadrifoglio F., Manzini G., Vasser M., Dinkelspiel K., Crea R. Conformational stability of alternating d (CG) oligomers in high salt solution. Nucleic Acids Res. 1981 May 11;9(9):2195–2206. doi: 10.1093/nar/9.9.2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirdivant S. M., Kłysik J., Wells R. D. Energetic and structural inter-relationship between DNA supercoiling and the right- to left-handed Z helix transitions in recombinant plasmids. J Biol Chem. 1982 Sep 10;257(17):10159–10165. [PubMed] [Google Scholar]
- Tait R. C., Rodriguez R. L., West R. W., Jr The rapid purification of T4 DNA ligase from a lambda T4 lig lysogen. J Biol Chem. 1980 Feb 10;255(3):813–815. [PubMed] [Google Scholar]
- Thamann T. J., Lord R. C., Wang A. H., Rich A. The high salt form of poly(dG-dC).poly(dG-dC) is left-handed Z-DNA: Raman spectra of crystals and solutions. Nucleic Acids Res. 1981 Oct 24;9(20):5443–5457. doi: 10.1093/nar/9.20.5443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Wang A. H., Quigley G. J., Kolpak F. J., Crawford J. L., van Boom J. H., van der Marel G., Rich A. Molecular structure of a left-handed double helical DNA fragment at atomic resolution. Nature. 1979 Dec 13;282(5740):680–686. doi: 10.1038/282680a0. [DOI] [PubMed] [Google Scholar]