Abstract
Objective
We hypothesize that time to initiate care and maturity of a treatment program impact on outcome of severely immuno-compromised patients with higher risk of mortality.
Design
We conducted a retrospective cohort analysis at the Perinatal HIV Research Unit Adult ART clinic, Soweto, South Africa.
Methods
Eligibility criteria for this analysis were: attendance for minimum one visit between August 2004 and August 2010, age >18 years, CD4 count < 50 cells/mm3 and ART-naïve at screening. We followed participants up to one year after ART initiation. We defined years 2004-2007 and 2008-2010 as the early and late eras respectively. Chi-square test and survival analysis methods were used for mortality comparisons between eras.
Results
Of 2357 patients eligible for antiretroviral treatment, 395 (17%) had CD4 counts < 50 cells/mm3 and ART-naïve at screening. Overall 261 (66%) were women. Patients had similar median age (35 vs. 33.5 years, p=0.08), time to HAART initiation (7 days, p=0.18) and baseline CD4 count (20 vs. 23 cells/mm3, p=0.5) between eras. Overall 63 (16%) patients died in their first year of treatment (2 per 100 person-months) and the main cause of death was tuberculosis (n=23, 37%). The proportion of deaths (52/262 vs. 11/133, p=0.003) and time to death from enrolment (logrank p=0.04) were significantly different between eras.
Conclusion
Mortality decreased as the ART program matured in Soweto while time to initiation of treatment remained similar in both eras. Because ART guidelines were consistent during both eras, it is possible that with time, management of patients improved as expertise was gained.
Introduction
The provision of free antiretroviral therapy (ART) services in sub-Saharan Africa has increased the number of people accessing treatment, consequently increasing life expectancy[1-3]. Unfortunately the triumphs of ART programs are eroded by early mortality rates on treatment observed especially in patients who present with severe immune compromise[4-7].
South Africa has the largest number of HIV-infected people and the largest ART program worldwide. Many patients present to the national treatment program at an advanced state of immune compromise: in one program in Khayelitsha, South Africa, the median CD4 count at entry was 43 cells/ul[8]. Multiple issues make the management of individuals with advanced immune suppression more difficult especially in a resource-constrained healthcare system: the increased risk of opportunistic illnesses which may be both difficult and expensive to diagnose and treat, the increased risk of immune reconstitution inflammatory syndrome, and the timing of ART initiation in the context of opportunistic disease.
Lower CD4 counts signify advancing immune compromise and are associated with an increased risk of morbidity and mortality[6,9-11]. CD4 counts less than 50cells/mm3 are associated with a higher risk of opportunistic illnesses such as Pneumocystis jirovecii pneumonia (PJP), cryptococcal meningitis, cytomegalovirus retinitis, central nervous system lymphoma and deaths from tuberculosis[12].
Many individuals with advanced immune compromise present after they develop an acute opportunistic illness, and timing ART initiation in this context can be delicate when considering the risks of drug-drug interactions, additive adverse events, pill burden and immune reconstitution inflammatory syndrome. In more recent years however, evidence has shown that early ART initiation in the presence of an opportunistic infection can reduce disease progression and mortality[13-16].
The first South African ART guideline was published in 2004, and it remained in effect until 2010. During 2004-2010, ART initiation was permitted at CD4 cell count less than 200 cells/mm3. Additionally, adults with CD4 counts less than 50 cells/mm3 were targeted as a group for fast-track ART initiation within two weeks. National guidelines on timing of ART initiation for tuberculosis patients were that those with CD4 counts less than 50 cells/mm3 should be initiated within 2 weeks, and those with higher CD4 counts should be started within 2 months[17,18].
Our study focuses on those who presented for care with advanced immune compromise i.e. CD4 counts less than 50 cells/mm3, to assess the implementation of the national guideline and provide information on mortality outcomes.
Methods
Study design
This retrospective cohort analysis investigated median time to ART initiation and mortality in adult patients with screening CD4 count < 50 cells/mm3.
Study setting
The study was conducted at the adult HIV Treatment Access clinic at the Perinatal HIV Research Unit (PHRU), Soweto, South Africa. The clinic has received funding from USAID/PEPFAR since 2004. It was accredited in 2010 by the National Department of Health as a Comprehensive Care Management and Treatment (CCMT) clinic. By the end of August 2010, 2357 HIV-infected individuals had been enrolled to receive treatment and care at the PHRU Treatment Access clinic.
Patients
Eligibility criteria for this study were: attendance at the PHRU Treatment Access clinic for at least one visit between August 2004 and August 2010, CD4 count < 50 cells/mm3 at screening, 18 years or older and ART-naïve at screening (note: women who used short-course ART for prevention of vertical transmission were not excluded).
Definitions
Screening was defined as the first clinic visit. Time to ART initiation was defined as the number of days between screening and ART initiation. Delayed ART initiation was defined as ART initiation beyond (but not including) 14 days. The early era was defined as patients screened and initiated between August 2004-2007 and the late era was defined as those patients screened and initiated between August 2008-2010. There were no treatment guideline changes between the two eras.
Outcomes
We assessed two main outcomes within the first year of treatment: treatment initiation and mortality.
Measures
We conducted medical record reviews that focused on the time period between screening and one year post ART initiation. The following variables were collected: gender; date of birth; medical history including referral source, date of screening, CD4 count at screening, date of ART initiation, date of death or date of one year visit (whichever occurred first).
Reasons for delayed initiation (defined in this analysis as time to initiation more than 14 days) were collated from medical records and classified into: co-morbid illnesses including hospitalisations; patient-related factors; institution-related and other reasons. Causes of death were collated from medical records which included hospital records where applicable or verbal autopsies from patient family members.
Ethical approval
Written consent was obtained from each participant for their information to be stored in the clinic database and used for research purposes. Ethical approval for this study was obtained from the University of the Witwatersrand Human Research Ethics Committee (Medical).
Statistical analysis
Continuous data was analysed descriptively through medians and interquartile ranges overall and by gender. Data was further split into the time periods 2004-2007 and 2008-2010 and continuous variables were compared non-parametrically using the Kruskall-Wallis test. Similarly comparisons were made between those initiating treatment within and after 14 days. Categorical comparisons between gender and eras were performed by chi-square analysis.
Kaplan-Meier methods and the log-rank p-value were used to compare time to death from enrolment and time of starting ART by gender and the periods 2004-2007 and 2008-2010. Univariate and multivariate Cox regression analysis controlling for gender, age and baseline CD4 count were used to determine predictors of mortality overall and in both eras. Hazard ratios, their 95% confidence intervals and p-values were used to determine significance. All statistical analysis was performed using SAS 9.3 and assuming a two-sided level of confidence.
Results
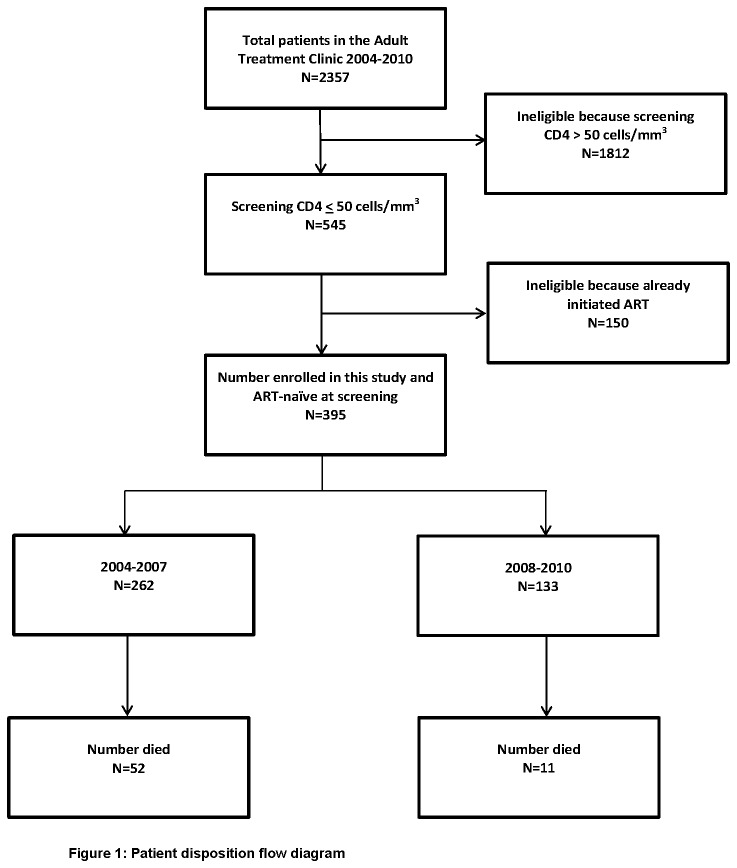
A total of 2357 patients registered to receive treatment in the adult Treatment Access clinic at PHRU, in the six year period between August 2004 and August 2010. Of these, 395 (17%) met eligibility criteria for our analysis (Figure 1). Of those enrolled in this study, 262 and 133 were in the periods 2004-2007 and 2008-2010 respectively. Most of the participants were women (n=261, 66%). The median age at enrolment for men was 36.9 (IQR: 31.0-41.5) and 33.5 (IQR: 27.9-38.3) years for women (p-value < 0.0001). Males were significantly older than females at enrolment in both periods. The overall median CD4 count and days to ART initiation were similar between males and females (Table 1). The median (IQR) duration of follow-up was 11.1 months (5.6-12.1); it was 11.2 (10.4-12.6) in era one and 10.0 (0.9-11.4) in the second era. No participants were lost to follow-up in the one year follow-up duration of the study; the only terminations were from deaths.
Figure 1. Patient disposition flow diagram.
Table 1. Patient characteristics at enrolment into the treatment access program.
| Variable | Overall | Males | Females | P-Value |
|---|---|---|---|---|
| Overall | ||||
| Number enrolled | 395 | 134 | 261 | |
| Median age in years at screening (IQR) | 34.4 (29.3-39.8) | 36.9 (31.0-41.5) | 33.5 (27.9-38.3) | <.0001 |
| Median CD4 count at screening (IQR) | 22.0 (8.0-37.0) | 23.5 (9.0-38.0) | 20.0 (8.0-36.0) | 0.4192 |
| Median days to ARV initiation (IQR) | 7.0 (6.0-12.0) | 7.0 (6.0-11.0) | 7.0 (6.0-13.0) | 0.7335 |
| 2004-2007 | ||||
| Number enrolled | 262 | 86 | 176 | |
| Median age in years at screening (IQR) | 35.0 (29.7-40.3) | 36.7 (31.4-41.1) | 34.0 (28.2-39.4) | 0.0094 |
| Median CD4 count at screening (IQR) | 20.0 (8.0-36.0) | 19.5 (7.0-37.0) | 20.0 (8.0-35.5) | 0.9723 |
| Median days to ARV initiation (IQR) | 7.0 (6.0-11.0) | 6.0 (5.0-8.0) | 7.0 (6.0-13.0) | 0.0645 |
| 2008-2010 | ||||
| Number enrolled | 133 | 48 | 85 | |
| Median age in years at screening (IQR) | 33.5 (28.8-38.9) | 37.6 (31.0-43.7) | 32.4 (27.6-35.9) | 0.0016 |
| Median CD4 count at screening (IQR) | 23.0 (10.0-38.0) | 25.5 (11.5-40.0) | 22.0 (9.0-37.0) | 0.2203 |
| Median days to ARV initiation (IQR) | 7.0 (6.0-13.0) | 9.0 (7.0-17.5) | 7.0 (6.0-12.0) | 0.0743 |
A total of 323 (82%) patients initiated treatment within 14 days of enrolment. There was no significant difference in the ages of those initiating ART within and after 14 days and by gender (Table 2). The median time of initiating treatment was 6 (IQR: 5-8) and 28 (IQR: 18-62.5) days (p<0.0001) for those initiating within and after 14 days respectively. The median baseline CD4 count was similar overall and in both periods in those initiating within and after 14 days. Further comparison between periods is presented in Table 3.
Table 2. Time of initiating treatment.
| Variable | Initiate ARVs within 14 days of screening | Initiate ARVs after 14 days of screening | p-value |
|---|---|---|---|
| Overall | |||
| Number (%) | 323 (82) | 72 (18) | |
| Age (IQR) | 34.5 (29.7-40.5) | 33.8 (28.1-37.9) | 0.32 |
| Gender | |||
| Male (%) | 110 (34) | 24 (33) | 0.99 |
| Female (%) | 213 (66) | 48 (67) | |
| Median days to initiation (IQR) | 6 (5-8) | 28 (18-62.5) | <0.0001 |
| Median baseline CD4 (IQR) | 21 (8-37) | 24 (10-35) | 0.84 |
| Deaths (%) | 49 (15) | 14 (19) | 0.38 |
| 2004-2007 | |||
| Number (%) | 218 (83) | 44 (17) | |
| Age (IQR) | 35.1 (30.3-40.5) | 34.4 (28.2-37.9) | 0.47 |
| Gender | |||
| Male (%) | 76 (35) | 10 (23) | 0.16 |
| Female (%) | 142 (65) | 34 (77) | |
| Median days to initiation (IQR) | 6 (5-7) | 28 (20-81.5) | <0.0001 |
| Median baseline CD4 (IQR) | 19.5 (7-35) | 24 (11-36.5) | 0.67 |
| Deaths (%) | 41 (18) | 11 (25) | 0.41 |
| 2008-2010 | |||
| Number (%) | 105 (79) | 28 (21) | |
| Age (IQR) | 33.5 (29.0-39.9) | 32.9 (28.1-37.8) | 0.60 |
| Gender | |||
| Male (%) | 34 (32) | 14 (34) | 0.97 |
| Female (%) | 71 (68) | 14 (66) | |
| Median days to initiation (IQR) | 7 (6-10) | 38.5 (17.5-49.5) | <0.0001 |
| Median baseline CD4 (IQR) | 23 (10-39) | 30.5 (9-34) | 0.78 |
| Deaths (%) | 8 (8) | 3 (11) | 0.70 |
Table 3. Comparison of variables between eras.
| Overall (N=395) | 2004-2007 (N=262, 66%) | 2008-2010 (N=133, 34%) | p-value | |
|---|---|---|---|---|
| Median Age in years (IQR) | 34.4 (29.3-39.8) | 35.0 (29.7-40.3) | 33.5 (28.8-38.9) | 0.08 |
| Gender | ||||
| Male (%) | 134 (34) | 86 (33) | 48 (36) | 0.57 |
| Female (%) | 261 (66) | 176 (67) | 85 (64) | |
| Median days to HAART initiation | 7 (6-12) | 7 (6-11) | 7 (6-13) | 0.18 |
| Initiate ARV within 14 days | ||||
| Yes (%) | 323 (82) | 218 (83) | 105 (79) | 0.3 |
| No (%) | 72 (18) | 44 (17) | 28 (21) | |
| Proportion initiating within 14 days (%) | 323 (82) | 218 (83) | 105 (79) | 0.3 |
| Median baseline CD4 (IQR) | 22 (8-37) | 20 (8-36) | 23 (10-38) | 0.25 |
| Deaths (%) | 63 (16) | 52 (20) | 11 (8) | 0.003 |
| Hospitalized | 22 | 16 | 6 | 0.5 |
In total, 63 (16%) patients died within one year of ART initiation at a rate of 2 (CI: 1.6-2.6) cases per 100 person-months (Table 2). There was no statistically significant difference in the proportion of deaths between those who initiated ART within versus after 14 days (15% vs. 19% respectively, p=0.38).Similarly the proportion of deaths was similar in those initiating within and after 14 days in both periods. There was a significantly (p=0.003) higher number of deaths in the period 2004-2007 (52, 20%) compared with 2008-2010 (11, 8%) (Table 3). The overall mortality rate was 2 (CI: 1.6-2.6) per 100-person months while it was 2 (CI: 1.5-2.6) and 1 (CI: 0.6-1.8) in 2004-2007 and 2008-2010 respectively. A total of 38 (60%) deaths occurred within the first three months of initiating treatment; 29 (56%) in 2004-2007 and 9 (82%) in 2008-2010 (p=0.11). At 6 months after initiating treatment, 42 and 10 deaths occurred in the first and second periods respectively (p=0.42).
The progression to death from enrolment was significantly higher in the first period compared to the second period (logrank p-value=0.04) while there was no difference in the overall time to death by gender (logrank p-value=0.2). There was no difference in the time to death from initiation of treatment stratified by gender (logrank p-value=0.2), period (logrank p-value=0.13) and time of ART initiation (logrank p-value=0.27).
In the overall multivariate Cox regression analysis (Table 4) adjusting for gender, age and baseline CD4 count, only those who had been hospitalized (HR: 3.896, CI: 1.935-7.847) predicted mortality. In the multivariate analysis stratified by period and adjusting for gender, age and baseline CD4 count, hospitalization (HR: 3.551, CI: 1.684-7.488) remained a significant predictor of mortality in the period 2004-2007. In the period 2008-2010, none of the variables (gender, age, time of ART initiation and hospitalization) predicted mortality.
Table 4. Overall Predictors of mortality from HAART initiation.
| Variable |
Univariate
|
Multivariate
|
||
|---|---|---|---|---|
| HR (CI) | p-value | HR (CI) | p-value | |
| Gender | ||||
| Male | 1.392 (0.840-2.307) | 0.1989 | 1.538 (0.919-2.573) | 0.1010 |
| Female | Ref | Ref | ||
| Age | ||||
| > 34 | 1.082 (0.660-1.774) | 0.7557 | 0.925 (0.553-1.546) | 0.7650 |
| <= 34 | Ref | Ref | ||
| Baseline CD4 | 0.982 (0.965-0.998) | 0.0323 | 0.982 (0.965-0.999) | 0.0374 |
| Time of ART Initiation | ||||
| Within 14 days | Ref | Ref | ||
| After 14 days | 1.398 (0.772-2.533) | 0.2686 | 1.438 (0.788-2.625) | 0.2369 |
| Hospitalized | ||||
| Yes | 4.119 (2.092-8.107) | <.0001 | 3.896 (1.935-7.847) | 0.0001 |
| No | Ref | Ref | ||
| Period | ||||
| 2008-2010 | Ref | Ref | ||
| 2004-2007 | 1.652 (0.862-3.168) | 0.1307 | 1.527 (0.785-2.972) | 0.2125 |
Causes of death were attributed to tuberculosis infection (n=23, 37%; 15 in first era and 8 in the second, p=0.9075) , unknown causes (n=16, 25%), gastroenteritis (n=7, 11%), respiratory tract infections (n=5, 8%), cardiac failure (n=3, 5%), cryptococcal meningitis (n=2, 3%), Kaposi’s sarcoma (n=2, 3%), Mycobacteria other than TB (n=2, 3%), epilepsy (n=1, 1.7%), Pneumocystis jirovecii (n=1, 1.7%), and sepsis (n=1, 1.7%). One hundred percent of deaths related to the AIDS diagnoses of cryptococcal meningitis, Kaposi’s sarcoma, Mycobacteria other than TB, and Pneumocystis jirovecii were in patients who had initiated ART within 14 days of screening.
Discussion
Our analysis reports on two major findings for immune compromised patients.
The first assesses guideline adherence for particularly immune compromised patients (CD4 < 50 cells/mm3) and demonstrates that expedited care is possible even in a busy South African setting serving a high percentage of immune compromised patients. Most (79%) received expedited ART well within the recommended 14 days at PHRU in Soweto, South Africa.
Second, we determined the effect of a maturing ART treatment program on mortality. Mortality was high in the first 3 to 6 months after starting ART. This high mortality is comparable to others reported in previous studies in Sub-Saharan Africa[19-22]. The proportion of deaths in the period 2004-2007 was significantly higher than that in 2008-2010. Variables predicting mortality in the early era were insignificant in the later era.
We observed that the mortality rate for immune compromised patients decreased as the ART program matured. It is plausible that treatment programs and healthcare professionals gain experience and skills in the management of advanced patients over time, potentially leading to better outcomes. This concurs with previously reported studies on predictors of mortality in HIV-infected patients in large ART treatment programs that show lower mortality outcomes as programs mature[4,23].
After controlling for age, baseline CD4 count, gender, time of initiating treatment and period, we found that previously hospitalized patients had a higher hazard of mortality. But when we determined predictors of mortality by period, hospitalization was predictive in the first period and none of the variables was predictive in the later period. As a treatment program matures, the risk of death declines. A possible explanation for this may be better management of patients as the treatment program matures leads to lower risk of death. Our findings concur with others reported elsewhere[4]. TB the main cause of death was similar between eras.
Because of limitations in our database, we could not include anemia, CDC staging, WHO staging and low BMI at baseline in our analysis, all of which are documented predictors of mortality. The lack of clinical disease data limits our ability to interpret our findings fully. It is possible that at the same CD4 value, patients in the earlier era were sicker (e.g. lower body mass index, more advanced clinical disease, lower haemoglobin) and it is unknown whether there would still be a difference in mortality between the eras after adjusting for clinical variables. Data was also not available for staff changes between the two eras.
We found decreased mortality and a lower risk of death as an ART program matured in Soweto, South Africa. It is possible that this reflects an improvement in the management of patients presenting with advanced immune suppression over time
Mortality decreased as the ART program matured in Soweto while time to initiation of treatment remained similar in both eras. Because ART guidelines were consistent during both eras, it is possible that with time, management of patients improved as expertise was gained.
Acknowledgments
We gratefully acknowledge Mr Niven Naicker for data management; Mr Thomas Mogale for data collation; the clinic staff and the patients.
Funding Statement
The authors gratefully acknowledge the South African National Department of Health andUSAID/PEPFAR for funding the adult treatment clinic. The first author is a fellow of the Consortium for Advanced Research Training in Africa (CARTA) and has received funding support from the Canada-Africa Prevention Trials Network (CAPT). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. UNAIDS (2010) Global report: UNAIDS report on the global AIDS epidemic.
- 2. UNAIDS (2011) World AIDS Day Report.
- 3. WHO (2011) Global HIV/AIDS. Response. [Google Scholar]
- 4. Hoffmann CJ, Fielding KL, Johnston V, Charalambous S, Innes C et al. (2011) Changing predictors of mortality over time from cART start: implications for care. J Acquir Immune Defic Syndr 58: 269-276. doi: 10.1097/QAI.0b013e31823219d1. PubMed: 21876447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kouanda S, Meda IB, Nikiema L, Tiendrebeogo S, Doulougou B et al. (2012) Determinants and causes of mortality in HIV-infected patients receiving antiretroviral therapy in Burkina Faso: a five-year retrospective cohort study. AIDS Care 24: 478-490. PubMed: 22148973. [DOI] [PubMed] [Google Scholar]
- 6. Mujugira A, Wester CW, Kim S, Bussmann S, Gaolathe T (2009) Patients with advanced HIV type 1 infection initiating antiretroviral therapy in Botswana: treatment response and mortality. AIDS Res Hum Retroviruses 25: 127-133. doi: 10.1089/aid.2008.0172. PubMed: 19239353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stringer JSA, Zulu I, Levy J, Stringer EM, Mwango A et al. (2006) Rapid scale-up of antiretroviral therapy at primary care sites in Zambia. JAMA 296: 782-793. doi: 10.1001/jama.296.7.782. PubMed: 16905784. [DOI] [PubMed] [Google Scholar]
- 8. Coetzee D, Hildebrand K, Boulle A, Maartens G, Louis F et al. (2004) Outcomes after two years of providing antiretroviral treatment in Khayelitsha, South Africa. AIDS 18: 887-895. doi: 10.1097/00002030-200404090-00006. PubMed: 15060436. [DOI] [PubMed] [Google Scholar]
- 9. Braitstein P, Brinkhof MW, Dabis F, Schechter M, Boulle A et al. (2006) Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet 367: 817-824. doi: 10.1016/S0140-6736(06)68337-2. PubMed: 16530575. [DOI] [PubMed] [Google Scholar]
- 10. Lawn SD, Myer L, Bekker LG, Wood R (2006) CD4 cell count recovery among HIV-infected patients with very advanced immunodeficiency commencing antiretroviral treatment in sub-Saharan Africa. BMC Infect Dis 6: 59. doi: 10.1186/1471-2334-6-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Smit C, Hallett TB, Lange J, Garnett G, de Wolf F (2008) Late entry to HIV care limits the impact of anti-retroviral therapy in The Netherlands. PLOS ONE 3: e1949. doi: 10.1371/journal.pone.0001949. PubMed: 18398473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jung AC, Paauw DS (1998) Diagnosing HIV-related disease: using the CD4 count as a guide. J Gen Intern Med 13: 131-136. doi: 10.1046/j.1525-1497.1998.00031.x. PubMed: 9502375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Abdool Karim SS, Naidoo K, Grobler A, Padayatchi N, Baxter C et al. (2011) Integration of antiretroviral therapy with tuberculosis treatment. N Engl J Med 365: 1492-1501. doi: 10.1056/NEJMoa1014181. PubMed: 22010915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Blanc FX, Sok T, Laureillard D, Borand L, Rekacewicz C et al. (2011) Earlier versus later start of antiretroviral therapy in HIV-infected adults with tuberculosis. N Engl J Med 365: 1471-1481. doi: 10.1056/NEJMoa1013911. PubMed: 22010913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Havlir DV, Kendall MA, Ive P, Kumwenda J, Swindells S et al. (2011) Timing of antiretroviral therapy for HIV-1 infection and tuberculosis. N Engl J Med 365: 1482-1491. doi: 10.1056/NEJMoa1013607. PubMed: 22010914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zolopa A, Andersen J, Powderly W, Sanchez A, Sanne I et al. (2009) Early antiretroviral therapy reduces AIDS progression/death in individuals with acute opportunistic infections: a multicenter randomized strategy trial. PLOS ONE 4: e5575. doi: 10.1371/journal.pone.0005575. PubMed: 19440326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. DoH (2004) South Africa Antiretroviral Treatment Guidelines. In: Health DO. [Google Scholar]
- 18. DoH (2010) South Africa Antiretroviral Treatment Guidelines. In: Health DO. [Google Scholar]
- 19. Alemu AW, Sebastián MS (2010) Determinants of survival in adult HIV patients on antiretroviral therapy in Oromiyaa, Ethiopia. Glob Health Action 3: 5398 PubMed: 21042435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Erikstrup C, Kallestrup P, Zinyama R, Gomo E, Mudenge B et al. (2007) Predictors of mortality in a cohort of HIV-1 infected adults in rural Africa. Journal of Acquired Immune Deficiency Syndromes 44: 478-483. doi: 10.1097/QAI.0b013e318032bbcd. [DOI] [PubMed] [Google Scholar]
- 21. Johannessen A, Naman E, Ngowi BJ, Sandvik L, Matee MI et al. (2008) Predictors of mortality in HIV-infected patients starting antiretroviral therapy in a rural hospital in Tanzania. BMC Infect Dis 8: 52. doi: 10.1186/1471-2334-8-52. PubMed: 18430196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ojikutu BO, Zheng H, Walensky RP, Lu Z, Losina E et al. (2008) Predictors of mortality in patients initiating antiretroviral therapy in Durban, South Africa. S Afr Med J 98: 204-208. PubMed: 18350223. [PMC free article] [PubMed] [Google Scholar]
- 23. Russell EC, Charalambous S, Pemba L, Churchyard GJ, Grant AD et al. (2010) Low haemoglobin predicts early mortality among adults starting antiretroviral therapy in an HIV care programme in South Africa: a cohort study. BMC Public Health 10: 433. doi: 10.1186/1471-2458-10-433. PubMed: 20653940. [DOI] [PMC free article] [PubMed] [Google Scholar]