Abstract
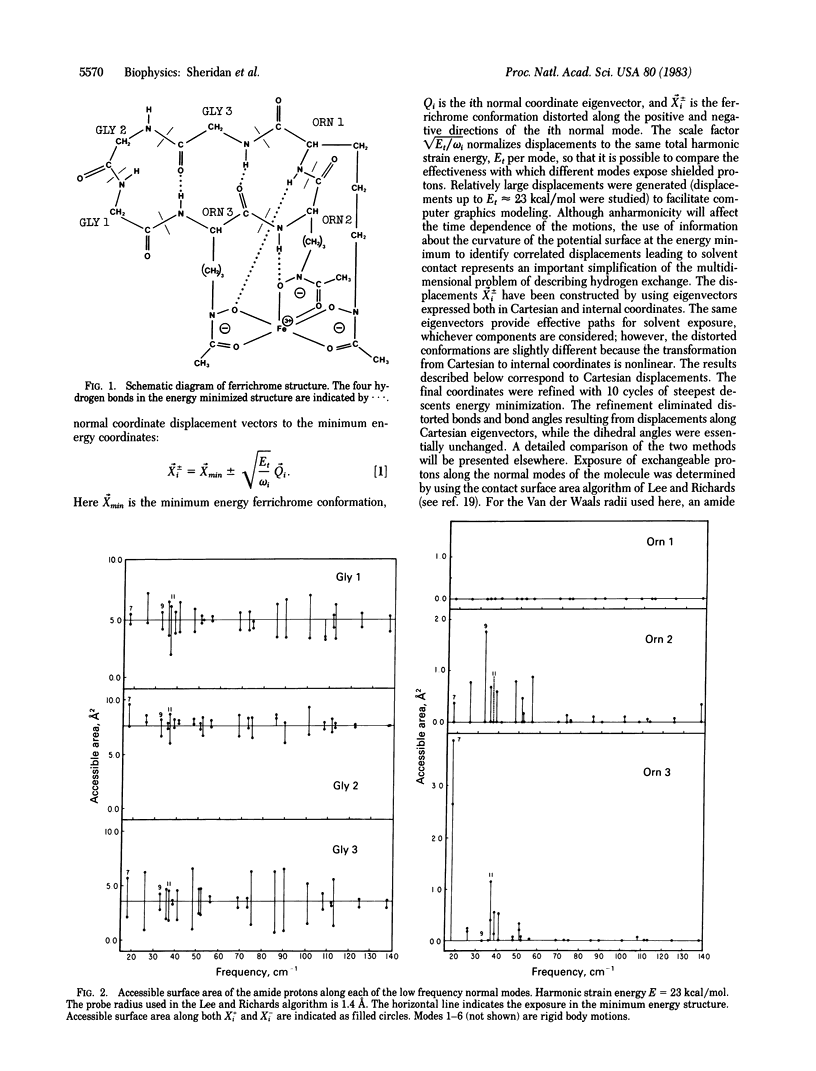
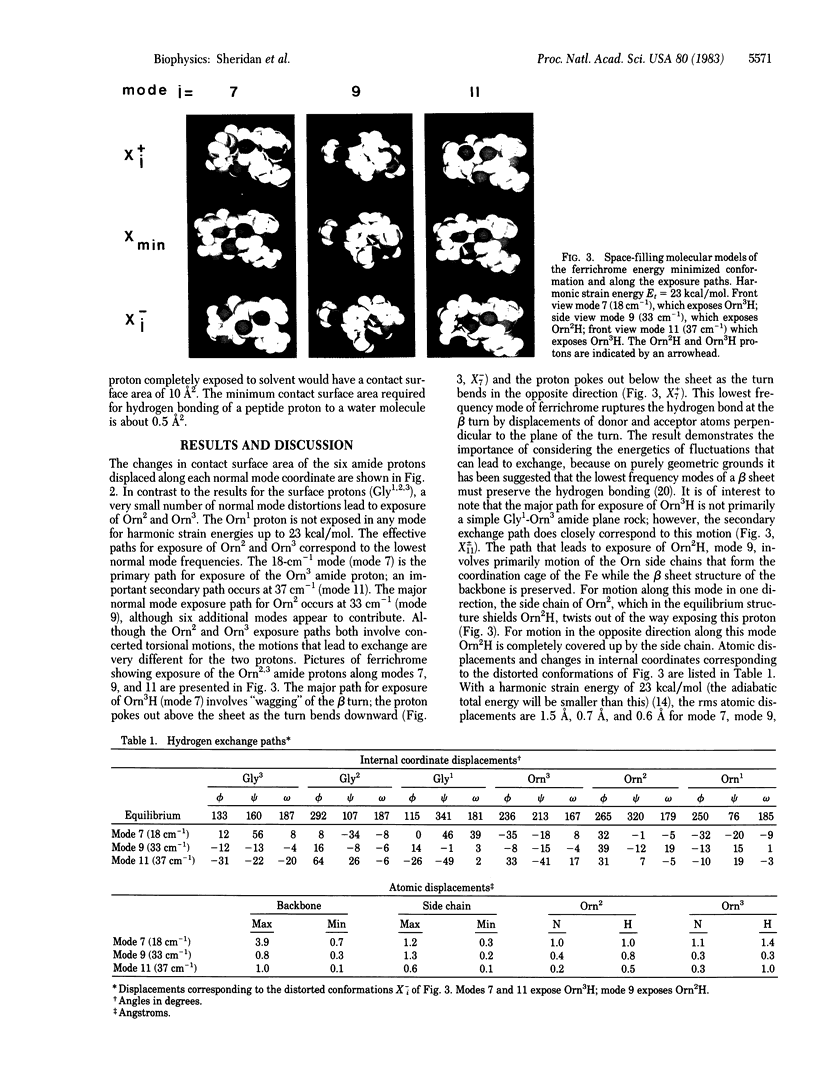
Possible paths for exposure to solvent and hydrogen exchange of the amide protons of ferrichrome, a cyclic hexapeptide, are examined. The paths are obtained from calculations of the vibrational normal modes of ferrichrome and correspond to low energy atomic displacements away from the local minimum in the multidimensional conformational space of the molecule. Exposure of exchangeable groups along the normal modes was determined by using the solvent accessible surface area algorithm of Lee and Richards. Three of the exchangeable protons (Gly1,2,3,) are largely exposed to solvent in the x-ray structure while the remaining three exchangeable protons of the ornithines are totally shielded from solvent. A very small number of normal mode displacements are found to expose the Orn2 and Orn3 amide groups while the Orn1 amide proton remains shielded from solvent for all the paths studied. The effective paths for exposure of Orn2 and Orn3 correspond to the lowest frequency (≈18 cm-1) motions. The paths are characterized in terms of the magnitude and energy of atomic displacements, correlated changes in dihedral angles, and the resulting changes in exposure and hydrogen bonding of exchangeable groups.
Keywords: peptide surface accessibility, atomic fluctuations
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Emery T. F. Slow tritium-hydrogen exchange in some cyclic peptide chelates. Biochemistry. 1967 Dec;6(12):3858–3866. doi: 10.1021/bi00864a032. [DOI] [PubMed] [Google Scholar]
- Englander S. W., Calhoun D. B., Englander J. J., Kallenbach N. R., Liem R. K., Malin E. L., Mandal C., Rogero J. R. Individual breathing reactions measured in hemoglobin by hydrogen exchange methods. Biophys J. 1980 Oct;32(1):577–589. doi: 10.1016/S0006-3495(80)84991-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englander S. W., Downer N. W., Teitelbaum H. Hydrogen exchange. Annu Rev Biochem. 1972;41:903–924. doi: 10.1146/annurev.bi.41.070172.004351. [DOI] [PubMed] [Google Scholar]
- Gelin B. R., Karplus M. Side-chain torsional potentials: effect of dipeptide, protein, and solvent environment. Biochemistry. 1979 Apr 3;18(7):1256–1268. doi: 10.1021/bi00574a022. [DOI] [PubMed] [Google Scholar]
- Kossiakoff A. A. Protein dynamics investigated by the neutron diffraction-hydrogen exchange technique. Nature. 1982 Apr 22;296(5859):713–721. doi: 10.1038/296713a0. [DOI] [PubMed] [Google Scholar]
- Levy R. M., Perahia D., Karplus M. Molecular dynamics of an alpha-helical polypeptide: Temperature dependence and deviation from harmonic behavior. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1346–1350. doi: 10.1073/pnas.79.4.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás M., Klein M. P., Neilands J. B. The solution conformation of the ferrichromes. IV. pH dependence of the individual slow amide hydrogen-deuterium exchange in alumichrome. J Biol Chem. 1973 Feb 10;248(3):915–923. [PubMed] [Google Scholar]
- Llinás M., Klein M. P., Neilands J. B. The solution conformation of the ferrichromes. V. The hydrogen exchange kinetics of ferrichrome analogues; the conformational state of the peptides. J Biol Chem. 1973 Feb 10;248(3):924–931. [PubMed] [Google Scholar]
- Noguti T., Go N. Collective variable description of small-amplitude conformational fluctuations in a globular protein. Nature. 1982 Apr 22;296(5859):776–778. doi: 10.1038/296776a0. [DOI] [PubMed] [Google Scholar]
- Richards F. M. Areas, volumes, packing and protein structure. Annu Rev Biophys Bioeng. 1977;6:151–176. doi: 10.1146/annurev.bb.06.060177.001055. [DOI] [PubMed] [Google Scholar]
- Salemme F. R. Cooperative motion and hydrogen exchange stability in protein beta-sheets. Nature. 1982 Oct 21;299(5885):754–756. doi: 10.1038/299754a0. [DOI] [PubMed] [Google Scholar]
- Smith J. A., Pease L. G. Reverse turns in peptides and proteins. CRC Crit Rev Biochem. 1980;8(4):315–399. doi: 10.3109/10409238009105470. [DOI] [PubMed] [Google Scholar]
- Wedin R. E., Delepierre M., Dobson C. M., Poulsen F. M. Mechanisms of hydrogen exchange in proteins from nuclear magnetic resonance studies of individual tryptophan indole NH hydrogens in lysozyme. Biochemistry. 1982 Mar 2;21(5):1098–1103. doi: 10.1021/bi00534a042. [DOI] [PubMed] [Google Scholar]
- Wlodawer A., Sjölin L. Hydrogen exchange in RNase A: neutron diffraction study. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1418–1422. doi: 10.1073/pnas.79.5.1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward C. K., Hilton B. D. Hydrogen exchange kinetics and internal motions in proteins and nucleic acids. Annu Rev Biophys Bioeng. 1979;8:99–127. doi: 10.1146/annurev.bb.08.060179.000531. [DOI] [PubMed] [Google Scholar]
- Woodward C. K., Hilton B. D. Hydrogen isotope exchange kinetics of single protons in bovine pancreatic trypsin inhibitor. Biophys J. 1980 Oct;32(1):561–575. doi: 10.1016/S0006-3495(80)84990-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wüthrich K., Wagner G., Richarz R., Braun W. Correlations between internal mobility and stability of globular proteins. Biophys J. 1980 Oct;32(1):549–560. doi: 10.1016/S0006-3495(80)84989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]