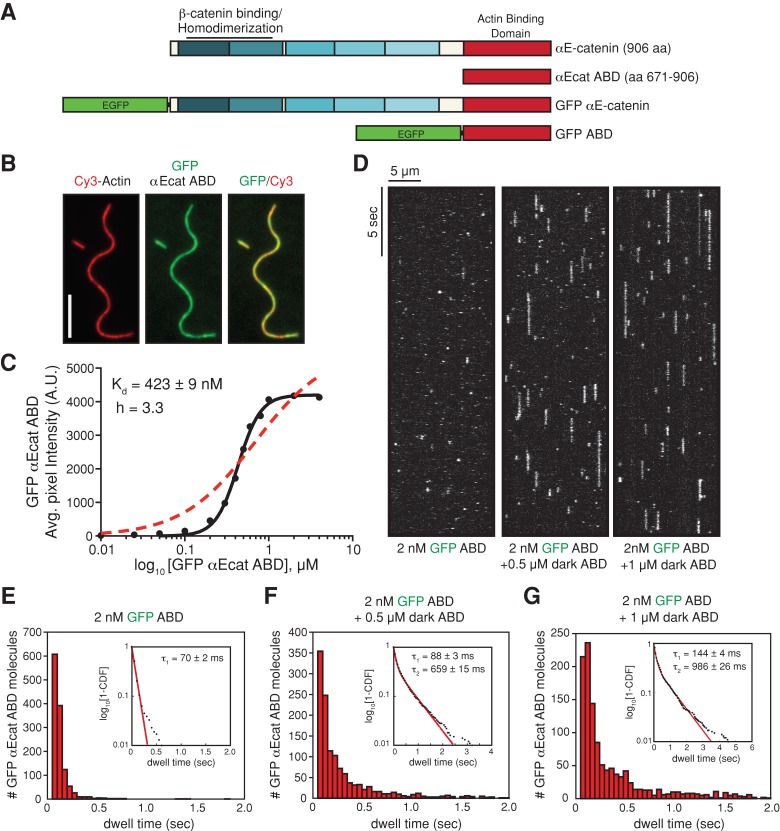
FIGURE 1:
αE-catenin ABD binds cooperatively to actin filaments. (A) αE-catenin is composed of an array of five four-helix bundles (blue-shaded boxes) and a C-terminal five-helix bundle (red box). The β-catenin/homodimerization region and actin-binding domain are marked. All αE-catenin constructs used in this study are defined. (B) Localization of 1 μM GFP αE-catenin ABD bound to phalloidin-stabilized filamentous actin (20% Cy3 labeled). Scale bar, 5 μm. (C) Average fluorescence signal of GFP αE-catenin ABD bound to single-actin filaments plotted against total concentration of GFP αE-catenin ABD. Each data point represents average GFP fluorescence per pixel measured over ≥100 μm of single actin filaments (≥2 TIRF flow chambers). Data were fitted to either a Hill equation (black, straight line) or a hyperbolic function (red, dashed line). (D) Kymographs showing 2 nM GFP αE-catenin ABD binding and dissociating from the sides of single actin filaments in the absence or presence of 0.5 or 1 μM dark αE-catenin ABD. (E–G) Histograms of 2 nM GFP αE-catenin ABD dwell times on filamentous actin in the absence (E) or presence (F) of 0.5 μM dark αE-catenin ABD or (G) 1 μM dark αE-catenin ABD. Inset, curve fit of the 1-cumulative distribution frequency: (E) single-exponential fit (τ1 = 70 ± 2 ms, n = 1244 molecules), (F) double-exponential fit (τ1 = 88 ± 3 ms [58%)], τ2 = 659 ± 15 ms [42%], n = 1289 molecules), and (G) double-exponential fit (τ1 = 144 ± 4 ms [64%], τ2 = 986 ± 26 ms [36%], n = 1210 molecules).