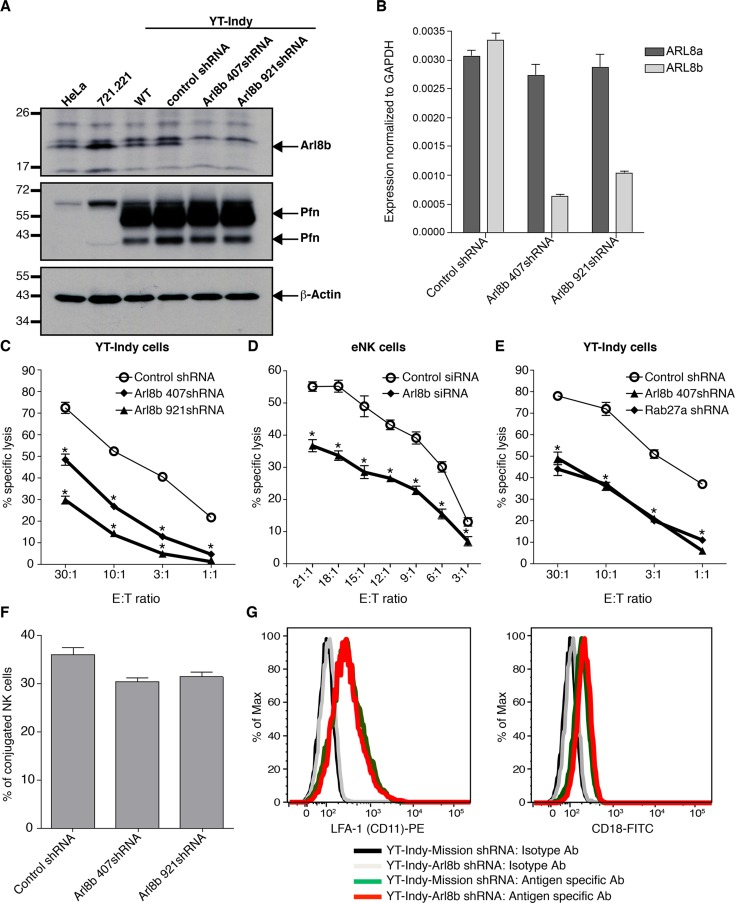
FIGURE 2:
Arl8b regulates NK cell cytotoxicity. (A) Stable silencing of Arl8b expression in NK cells using two distinct shRNA sequences (407 and 921). Left, Western blot analyses of Arl8b (top), perforin (middle), and actin (bottom) levels in YT-Indy (untreated) and YT-Indy stably expressing lentiviral vector–driven control shRNA, Arl8b 407 shRNA, or Arl8b 921 shRNA, as indicated. HeLa and 721.221 cell lysates were used as a negative control for perforin, and actin was used as a protein loading control. (B) qRT-PCR analyses of Arl8b and Arl8a levels in control shRNA–, Arl8b 407shRNA–, and Arl8b 921shRNAxexpressing YT-Indy cells. (C–E) Down-regulation of Arl8b impairs NK cytotoxicity. Cytotoxicity of YT-Indy cells stably expressing control shRNA– or Arl8b-specific shRNA-407 and -921 (C), control- or Arl8b siRNA–treated (72 h) primary human NK cells (D), and YT-Indy cells stable expressing control shRNA, Arl8b-specific shRNA, or Rab27a-specific shRNA (E) was tested against 721.221 target cells by 51Cr-release assay at various E:T ratios. Data show mean ± SEM from triplicates of one representative experiment of three performed; *p < 0.05. (F) Silencing of Arl8b does not affect conjugate formation. YT-Indy cells (control or Arl8b shRNA transduced) and 721.221 target cells were stained with PKH26 (Red Fluorescent Cell Linker) and PKH67 (Green Fluorescent Cell Linker), respectively. Labeled cells were coincubated at a 2:1 E:T ratio for 20 min, fixed in 4% PFA, and analyzed by flow cytometry. Events positive for red and green fluorescence were considered conjugates, and the percentage of conjugation was calculated as (red + green fluorescence/red fluorescence only) × 100. Data show mean ± SD of three independent experiments. Differences between groups were not significant. (G) Cell surface levels of CD11a and CD18 receptors remain unchanged in NK cells lacking Arl8b. YT-Indy cells (control or Arl8b shRNA transduced) were stained with isotype control, anti-CD11a-PE (left), or anti-CD18- fluorescein isothiocyanate (right) antibodies for 30 min on ice and analyzed by flow cytometry.