FIGURE 3.
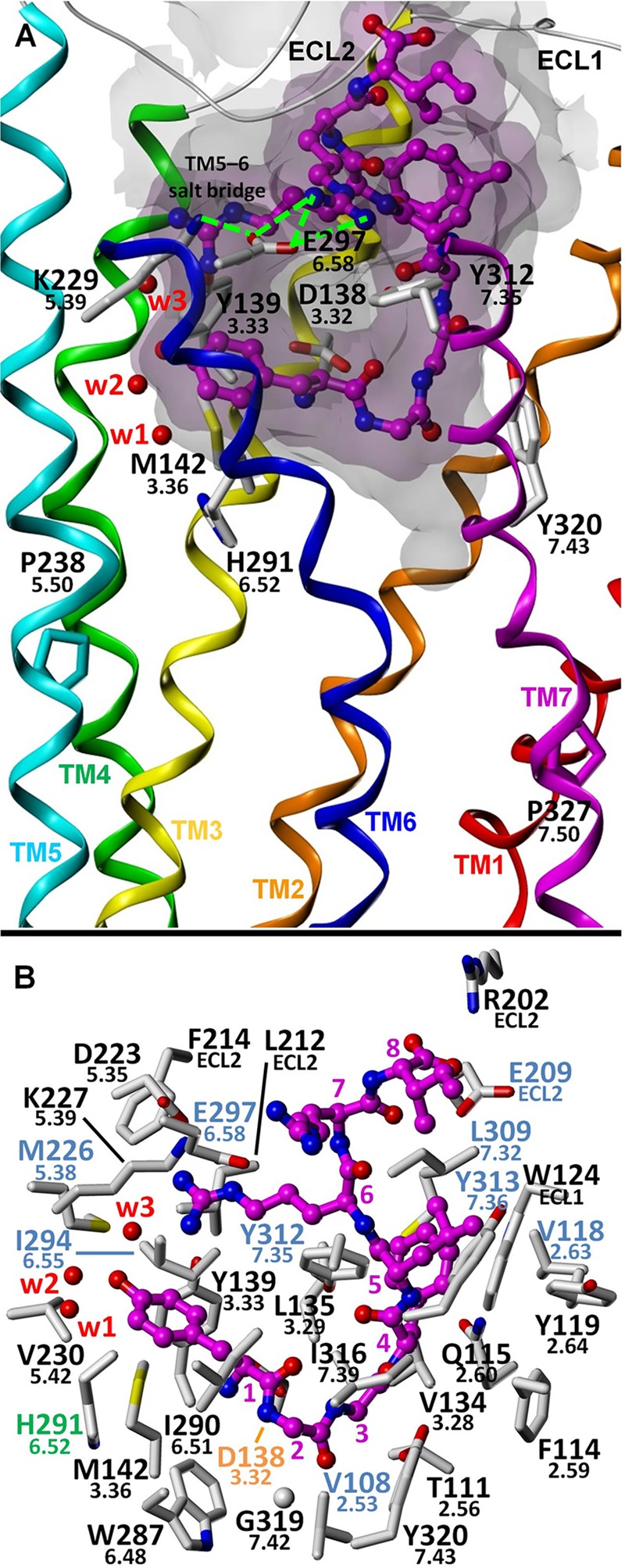
Proposed binding mode for dynorphin A(1–8). A, putative interaction of Arg7 of dynorphin A with Glu297(6.58) probably disturbs the stability of a salt bridge formed between Lys227(5.39) and Glu297(6.58) (potential hydrogen bonds are indicated with green lines). This salt bridge may serve to attenuate G protein-mediated signaling by limiting the ability of TM6 to adopt an active-like conformation, an effect that is probably analogous to the formation of the Ser222(5.43)–Asn344(6.55) hydrogen bond (and other nonpolar interactions) upon binding of the β-arrestin-selective agonist ergotamine at the 5-HT2B receptor. B, putative binding mode showing KOR amino acid residues within 4 Å of the ligand. Water molecules W1–W4 correspond to Wat1311, -1307, -1316, and -1314, respectively.
