FIGURE 4.
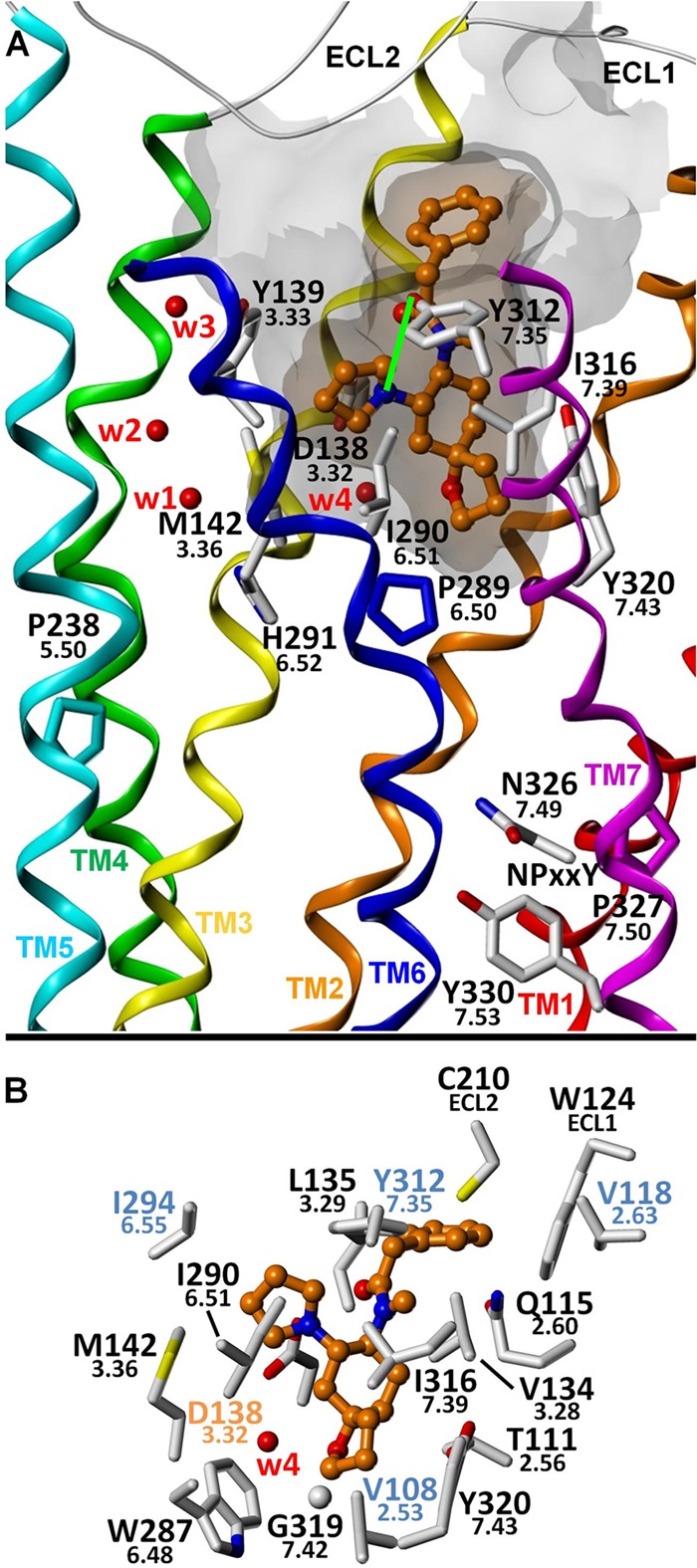
Proposed binding mode for U-69593. A, putative interaction of U-69593 with Ile316(7.39) and Ile290(6.51), binding site residues at positions that belong to a conserved network of key GPCR non-covalent interactions. Mutation of either Ile316 or Tyr320(7.43) one turn above significantly reduces the potency of all tested agonists, consistent with the importance of Ile316 for proper GPCR structure and function. Structurally, a likely explanation for this effect is that these mutations significantly hinder the ability of TM7 and its NPXXY motif to adopt a G protein-recognizing conformation. A hydrogen bond (shown as a green line) may be formed between Tyr312(7.35) and the pyrrolidine ring nitrogen atom in an alternate ring puckering conformation. B, putative binding mode showing KOR amino acid residues within 4 Å of the ligand. Water molecules W1–W4 correspond to Wat1311, -1307, -1316, and -1314, respectively.
