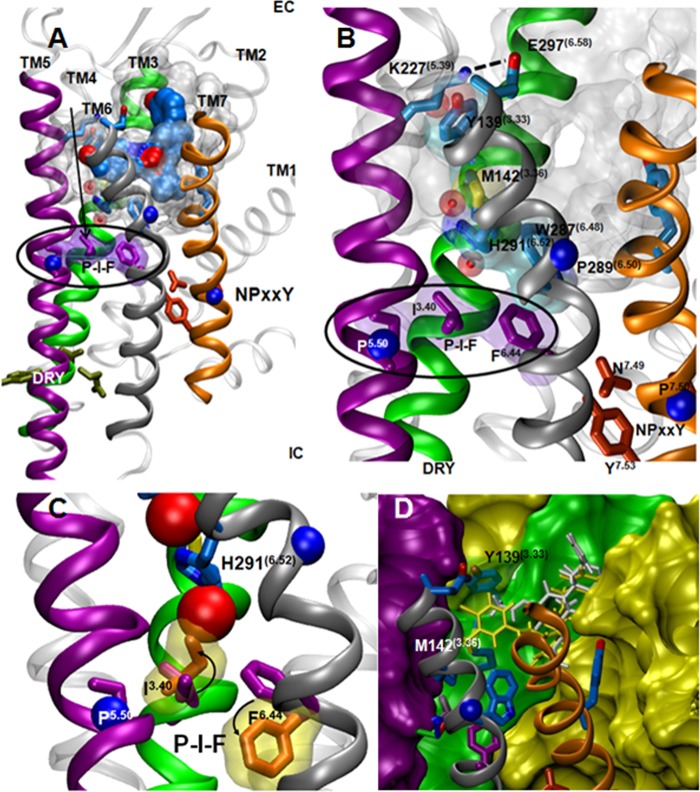
FIGURE 8.
Proposed activation pathways in the κ-opioid receptor. A, an overall view of the JDTic-bound KOR crystal structure with the important rotamer switch motifs (green residues, DRY; orange residues, NPXXY; purple residues, P-I-F motif). B, focus on the interface between TM3, TM5, and TM6, showing the proposed structural relationship between some of the tested positions and the P-I-F motif. C, the predicted changes in the rotameric state of Ile(3.40) and Phe(6.44) (orange) upon activation (based on the analysis of the active state P-I-F motif in several GPCR structures) introduce a collision with the water molecule trapped between Ile146 and His291 in the KOR crystal structure, suggesting that this water molecule is displaced upon KOR activation. D, a surface representation of the binding site of KOR. Mutagenesis of Tyr139 can create a cleft in the wall of the binding site. Salvinorin A and 1xx are represented by thin white and yellow sticks, respectively.