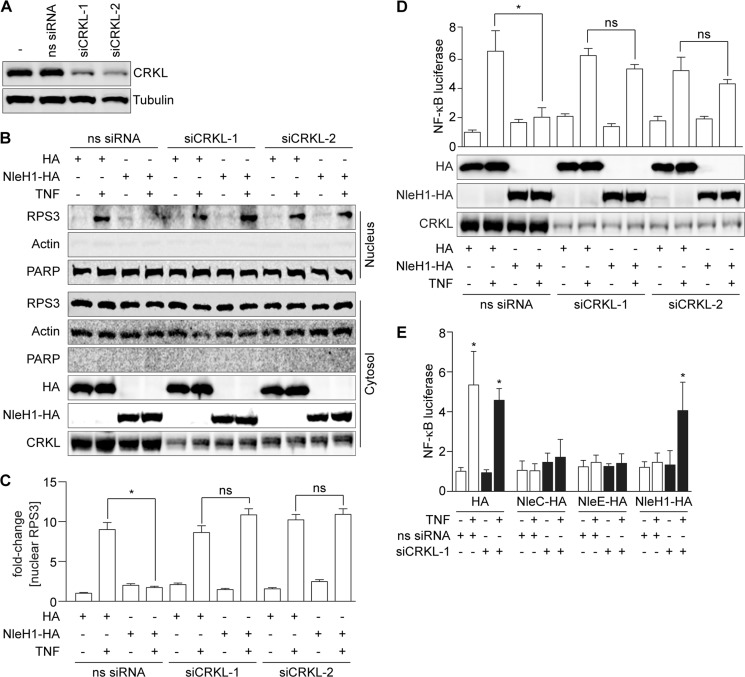
FIGURE 2.
CRKL is required for NleH1 to inhibit RPS3 nuclear translocation and NF-κB activity. A, CRKL knockdown with siRNAs. B, RPS3 nuclear translocation assay. HEK293T cells were cotransfected with NleH1-HA or an HA epitope control and with two independent CRKL siRNAs (siCRKL) or with a nonspecific siRNA (ns siRNA). After 48 h, cells were treated with TNF (50 ng/ml, 30 min), separated into nuclear and cytoplasmic fractions, and subjected to immunoblotting. PARP, poly(ADP-ribose) polymerase. C, quantification (n = 3) of the -fold change in nuclear RPS3 abundance after normalization to nuclear poly(ADP-ribose) polymerase abundance. Data are shown as means ± S.E. *, significant differences between the indicated pairwise comparisons (p < 0.05, t test). ns, not significant. D, NF-κB activity. HEK293T cells were cotransfected with NleH1, CRKL siRNA, a firefly luciferase construct driven by a consensus κB site, and a Renilla luciferase plasmid. Cells were stimulated with TNF (50 ng/ml, 30 min) after 48 h of transfection. NF-κB activity was determined by luciferase reporter assays. Data are shown as means ± S.E. of luciferase activity from three independent assays. *, significant differences between the indicated pairwise comparisons (p < 0.05, t test). The expression levels of CRKL, NleH-HA, and the HA control were determined by immunoblotting with anti-CRKL and anti-HA antibodies. E, NF-κB activity in HCT-8 cells. HCT-8 cells were cotransfected with CRKL siRNA and luciferase reporter plasmids in the presence or absence of NleC, NleE, or NleH1 and then treated with TNF (50 ng/ml, 30 min) 48 h after transfection. Data are shown as the means ± S.E. of luciferase activity from three independent assays. *, significant differences from unstimulated cells (p < 0.05, t test).