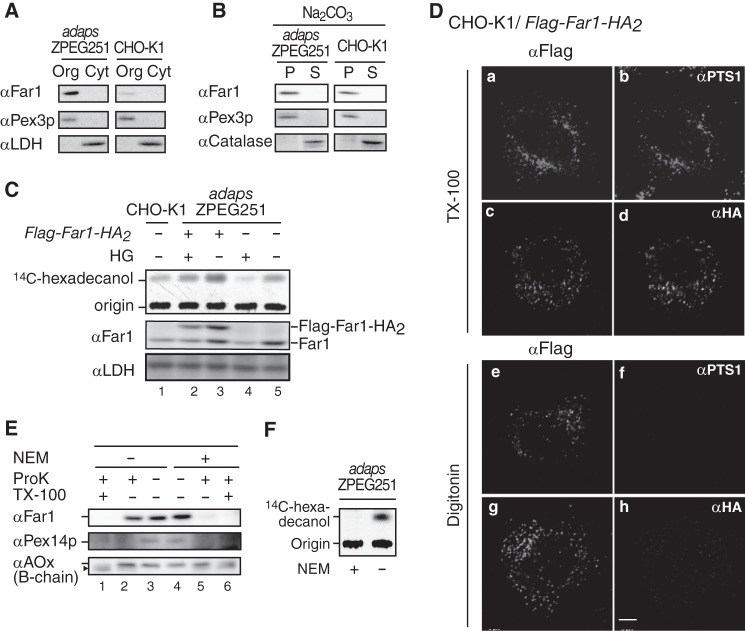
FIGURE 1.
Far1 is a tail-anchored type II peroxisomal membrane protein. A, organelle (Org) and cytosolic (Cyt) fractions prepared from ZPEG251, an ADAPS-defective CHO mutant cell line (adapsZPEG251), and CHO-K1 cells were assessed by Western blotting with antibodies against Far1, the peroxisomal membrane protein Pex3p, and lactate dehydrogenase. B, organelle fractions of ZPEG251 and CHO-K1 cells were treated with sodium carbonate, separated into membrane (P) and soluble (S) fractions, and subjected to immunoblotting with antibodies as indicated on the left. C, CHO-K1 cells (lane 1), ZPEG251 cells (lanes 4 and 5), and ZPEG251 cells stably expressing FLAG-Far1-HA2 (lanes 2 and 3) were cultured for 2 days in the presence (+) or absence (−) of HG. PNSs were prepared from the cells, and Far1 activity was assessed using [14C]palmitoyl-CoA as a substrate (top panel). Expression levels of endogenous Far1, FLAG-Far1-HA2, and lactate dehydrogenase were assessed by immunoblotting using antibodies against Far1 and lactate dehydrogenase (center and bottom panels, respectively). Origin, the spots where the extracted lipids were placed. D, transmembrane topology of FLAG-Far1-HA2. CHO-K1 cells transfected with FLAG-Far1-HA2 were fixed, treated with 1% TX-100 (a–d) or 25 μg/ml digitonin (e–h), and subjected to dual labeling with antibodies against FLAG (a and e) and PTS1 (b and f) or FLAG (c and g) and HA (d and h). Scale bar = 5 μm. E, PNS was prepared from ZPEG251 cells in the presence (+) or absence (−) of 1 mm NEM and subjected to proteinase K (ProK) (1 μg) digestion. The protease sensitivities of Far1, Pex14p, and acyl-CoA oxidase (αAOx) were assessed by immunoblotting with their corresponding antibodies. Note that Far1 was effectively degraded by proteinase K in the presence of NEM. The arrowhead indicates a cleavage product of AOx (42). F, PNS was prepared as described in E and assessed for Far activity with [14C]palmitoyl-CoA as a substrate in the presence (+) or absence (−) of NEM.