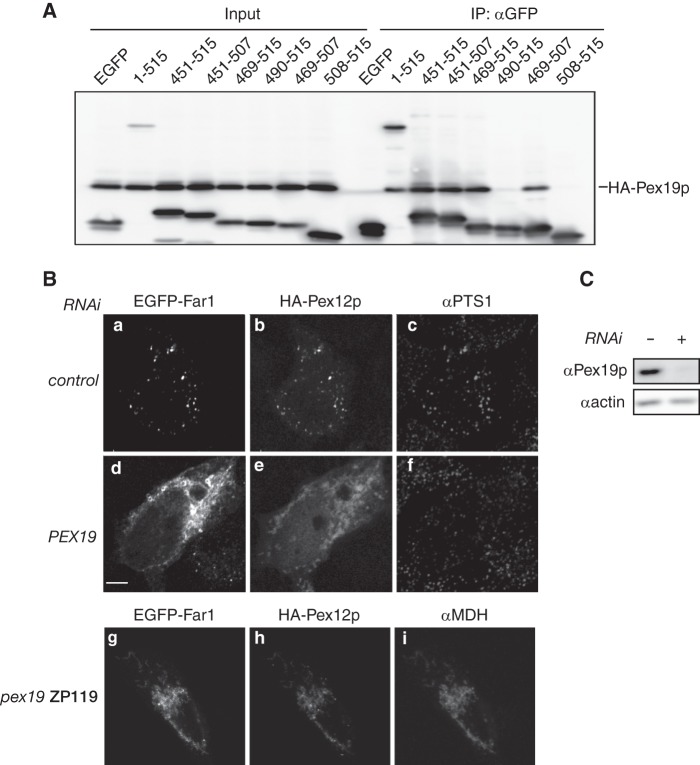
FIGURE 4.
Far1 is transported to peroxisomes in a Pex19p-dependent manner. A, EGFP fusion proteins containing full-length Far1 or a portion of the C terminus were coexpressed with HA-Pex19p in pex19 ZP119 cells for 14 h and subjected to immunoprecipitation with an anti-GFP antibody. EGFP-tagged Far1 proteins and HA-Pex19p were detected using antibodies against GFP and HA in the same membrane. Input, 2.5% of the total cell lysate used in each immunoprecipitation (IP). Note that HA-Pex19p bound all EGFP-tagged Far1 proteins that localized to peroxisomes in CHO-K1 cells (see Fig. 3B). B, HeLa cells were treated without (top panel) or with a dsRNA against PEX19 (center panel) for 72 h. EGFP-Far1 and HA-Pex12p (43) were coexpressed for the final 12 h of this treatment. EGFP-Far1 and HA-Pex12p were also coexpressed in pex19 ZP119 cells (bottom panel). The intracellular localizations of EGFP-Far1, HA-Pex12p, PTS1 proteins, and malate dehydrogenase were assessed by monitoring GFP fluorescence and labeling with antibodies against HA, PTS1, and malate dehydrogenase. Note that the localization of EGFP-Far1 and HA-Pex12p to peroxisomes was impaired when PEX19 expression was reduced, despite peroxisomes remaining intact in these cells as indicated by the punctate labeling of the anti-PTS1 antibody. Scale bar = 5 μm. C, depletion of Pex19p expression was confirmed by immunoblotting. Actin was used as a loading control.