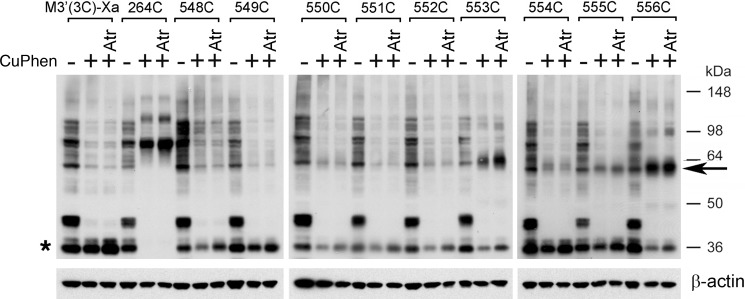
FIGURE 10.
Effect of CuPhen and atropine on the formation of disulfide cross-linked M3R dimers (H8 Cys mutants). Experiments were carried out in the same fashion as described in the legend to Fig. 9. In the absence of CuPhen, multiple immunoreactive bands can be detected, including a ∼60-kDa band that is similar in size to that of cross-linked M3R-M3R dimers (H8 Cys mutant M3Rs). However, it is unlikely that this band represents a cross-linked M3R dimer, because it is also observed with the M3′(3C)-Xa construct that lacks Cys residues available for disulfide cross-link formation. The Western blotting data indicate that CuPhen is required for efficient M3R-M3R disulfide cross-linking displayed by some of the H8 Cys mutant receptors. Moreover, with the exception of the 553C construct, atropine treatment (Atr; 1 μm) has no significant effect on the efficiency of M3R dimer formation. Interestingly, atropine selectively facilitates the formation of T553C mutant M3R dimers. The blots shown are representative of two independent experiments. Asterisk, putative receptor monomers; arrow, putative cross-linked receptor dimers.