FIGURE 11.
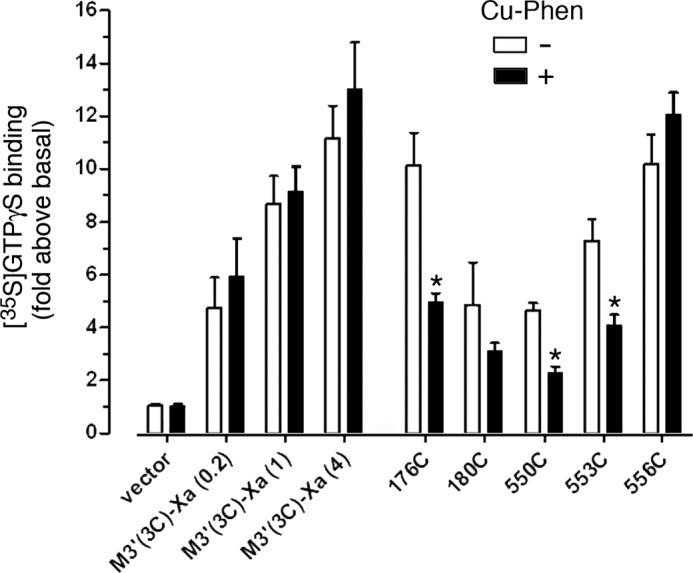
Several cross-linked mutant M3Rs show reduced G protein coupling efficacy. COS-7 cells were transfected with vector DNA, the M3′(3C)-Xa receptor, or the indicated M3′(3C)-Xa-derived Cys-substituted mutant constructs. Subsequently, cell membranes were incubated in the absence or presence of CuPhen (100 μm, 10 min at 37 °C). Cell membranes were then treated with the agonist carbachol (CCh; 1 mm) in the presence of [35S]GTPγS for 2 min at 30 °C. CCh-stimulated [35S]GTPγS binding was assessed by selective immunoprecipitation of [35S]GTPγS-labeled Gαq/11, using an anti-Gαq/11 antiserum. Because several mutant receptors were expressed at lower levels (Bmax) than the M3′(3C)-Xa construct (4 μg of DNA) from which they were derived, we also transfected cells with decreased amounts of M3′(3C)-Xa DNA (0.2 and 1 μg) to achieve a range of M3′(3C)-Xa Bmax values, similar to those observed with some of the mutant constructs (Table 3). Data are expressed as fold-increase in [35S]GTPγS binding above basal levels determined in the absence of agonist. Basal [35S]GTPγS binding activities were similar for the various mutant receptors. Data are given as mean ± S.E. of 3–5 independent experiments, each carried out in duplicate. *, p < 0.05, as compared with the corresponding non-CuPhen-treated samples.
