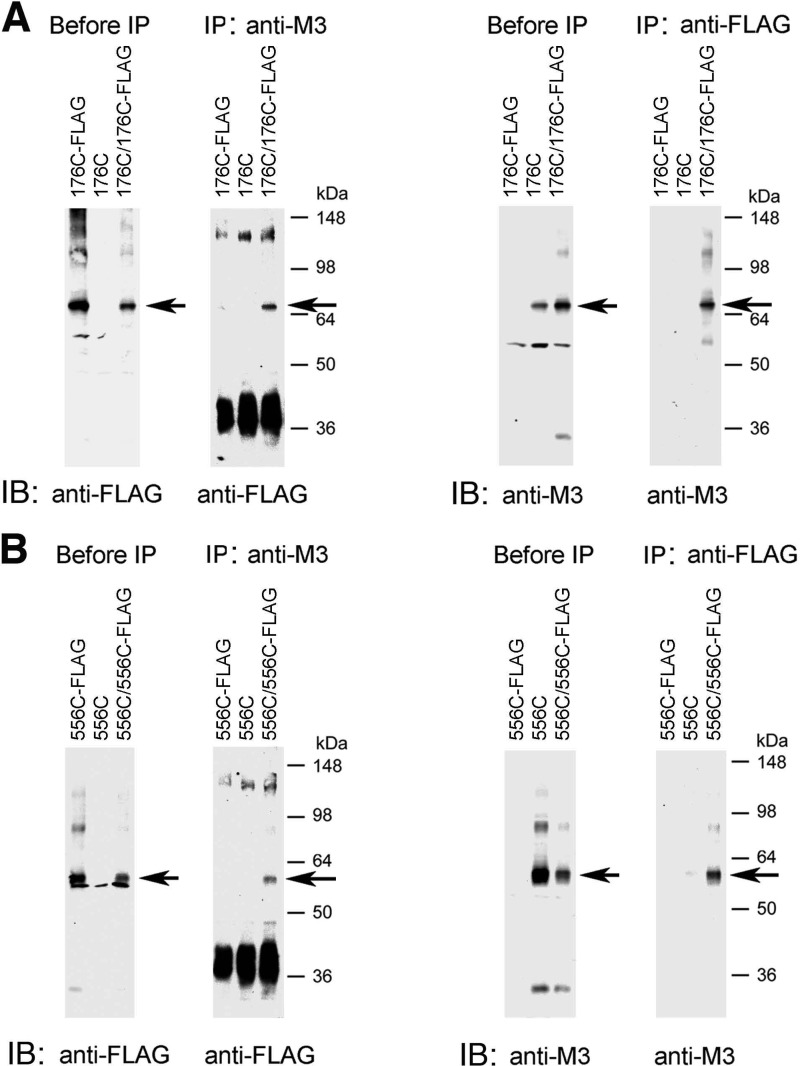
FIGURE 4.
Co-immunoprecipitation studies with representative mutant M3Rs confirming M3R homodimer formation. This figure shows co-immunoprecipitation data for two representative Cys-substituted M3R constructs, R176C (i2 loop) and T556C (H8). A, co-immunoprecipitation of cross-linked R176C and R176C-FLAG mutant M3Rs. B, co-immunoprecipitation of cross-linked T556C and T556C-FLAG mutant M3Rs. For these studies, we generated modified versions of the R176C and T556C constructs in which the C-terminal recognition sequence for the anti-M3R antibody (Fig. 1) was replaced with a FLAG tag, resulting in the R176C-FLAG and T556C-FLAG mutant M3Rs, respectively. Membrane samples prepared from COS-7 cells co-expressing R176C and R176C-FLAG (A) or T556C and T556C-FLAG (B) were treated for 10 min with 100 μm CuPhen as described under “Experimental Procedures.” Solubilized membrane proteins were then incubated with the anti-M3R polyclonal antibody (IP: anti-M3) or a monoclonal anti-FLAG antibody (IP: anti-FLAG). After this step, protein A-agarose was added, and the bound immunoreactive proteins were eluted and blotted with a monoclonal anti-FLAG antibody (immunoblot (IB): anti-FLAG) or the anti-M3R polyclonal antibody (IB: anti-M3) under non-reducing conditions. For comparison, Western blots of membrane proteins prior to the IP step are shown in the left panels (“before IP”). The blots shown are representative of two or three independent experiments. Arrows indicate putative cross-linked receptor dimers.