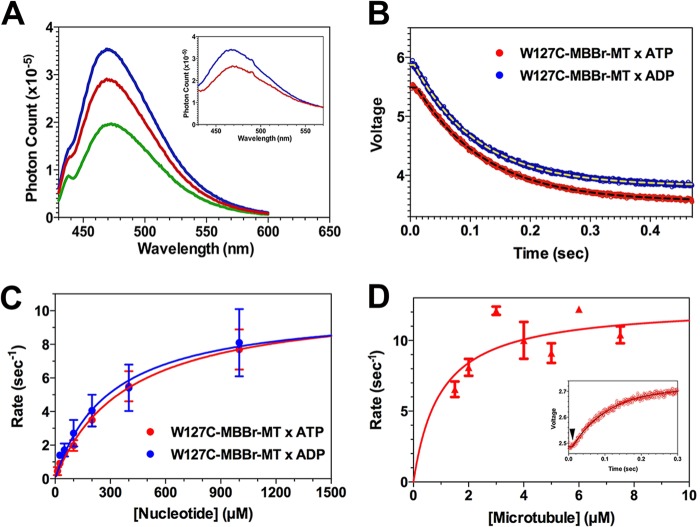
FIGURE 3.
Steady state and transient kinetics studies of mBBr-labeled W127C fluorescence. A, fluorescence emission spectra of mBBr-labeled W127C in rigor (blue), 2 mm AMPPNP (brown), and 2 mm ADP (green). Samples were excited at 395 nm. Conditions were as in Fig. 2. Inset, fluorescence emission of a complex of mBBr-labeled W127C + MTs in rigor (blue) and 2 mm AMPPNP (red), demonstrating that MT binding does not appreciably alter the relative quantum yields in the rigor and AMPPNP biochemical states. B, fluorescence transients produced by mixing a complex of mBBr-labeled W127C + MTs (1:4, MD·MT) with 800 μm ATP (red) or 800 μm ADP (blue). In both cases, the fluorescence decreases in a monophasic process following a brief lag. The jagged curve represents the measured photomultiplier voltage, and the dashed lines represent fits to a single exponential decay. C, plots of rate constants for the transients from the experiment in B versus [ATP] (red) or [ADP] (blue). Data represent the mean ± S.D. (error bars) The dependence on [ATP] or [ADP] was fit to a rectangular hyperbola, defining extrapolated maximum rate constants summarized in Table 1. Conditions were as in Fig. 2. D, kinetics of fluorescence enhancement of mBBr-labeled W127C·ADP produced by mixing with an excess of MTs + 2 mm ATP in the stopped flow instrument. The mBBr/MD molar ratio in this experiment was 0.89, and the sample was mixed with a 4–6-fold molar excess of polymerized tubulin. The resulting fluorescence enhancement consisted of a single monophasic increase following a lag of ∼10 ms (inset, arrowhead). The rate constant for this rising phase varied hyperbolically with [MT], defining a maximum extrapolated rate summarized in Table 1. Inset, fluorescence enhancement produced by mixing 2 μm mBBr-W127C·ADP with 8 μm polymerized tubulin. Error bars, S.D.