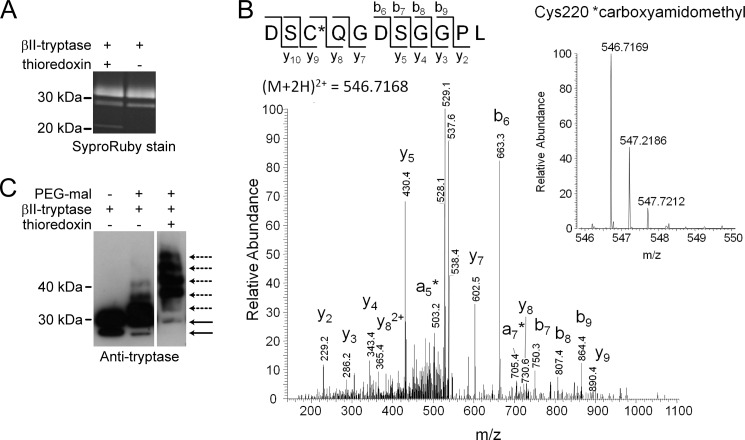
FIGURE 2.
The Cys220–Cys248 disulfide bond is reduced in βII-tryptase. A, the SYPRO Ruby-stained βII-tryptase used for mass spectrometry analysis. B, tandem mass spectrum of the DSCQGDSGGPL peptide from βII-tryptase showing the reduced form of Cys220 (underlined) labeled with the IAM adduct, carboxyamidomethyl. The accurate mass spectrum of the peptide is shown in the inset (observed [M + 2H]2+ = 546.7169 m/z; expected [M+2H]2+ = 546.7168 m/z). C, βII-tryptase (1 μm) was incubated without or with thioredoxin (1 μm) and then labeled with PEG-maleimide. The labeled sample was resolved on SDS-PAGE and immunoblotted for βII-tryptase. The solid lines are unlabeled β-tryptase, and the dotted lines are the PEGylated protein.