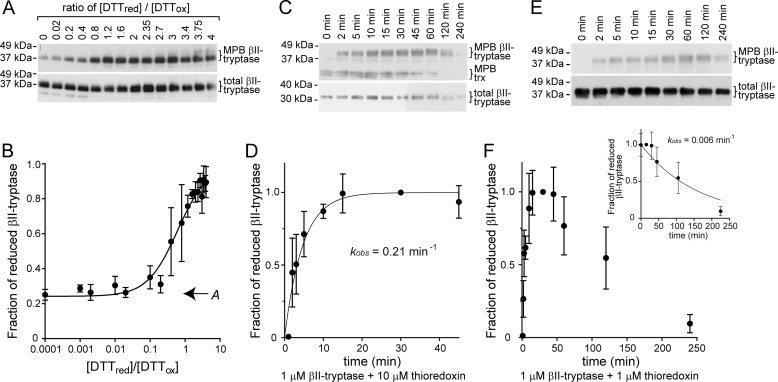
FIGURE 3.
Chemical properties of the βII-tryptase Cys220–Cys248 disulfide bond. A, βII-tryptase was incubated with 0.5 mm oxidized DTT (DTTox) and varying concentrations of reduced DTT (DTTred). Reduced protein was labeled with maleimide-biotin (MPB) and resolved on SDS-PAGE, and the MPB label was visualized by blotting with streptavidin-peroxidase. The positions of molecular mass standards in kDa are shown on the left. B, plot of the ratio of reduced to oxidized βII-tryptase as a function of the ratio of reduced to oxidized DTT from experiments of the type shown in A. The solid line represents the best nonlinear least squares fit of the data to Equation 1. The calculated equilibrium constant is 0.62 ± 0.13, and offset A is 0.24 ± 0.04 (indicated on graph). From the Nernst equation, the standard redox potential of the Cys220–Cys248 disulfide is −301 mV. The data points and errors are the means ± S.E. of three independent experiments. C, rate of reduction of the Cys220–Cys248 βII-tryptase disulfide bond by thioredoxin (trx). βII-Tryptase (1 μm) was incubated with thioredoxin (10 μm), and the reduced enzyme was detected by labeling with MPB at discrete times as described above. The positions of molecular mass standards in kDa are shown at the left. D, plot of the fraction of reduced βII-tryptase as a function of time from experiments of the type shown in C. The solid line represents the best nonlinear least squares fit of the data to a single exponential. The calculated kobs for the pseudo-first order reaction is 0.21 ± 0.05 min−1. The data points and errors are the means ± S.E. of three independent experiments. E, rate of oxidation of the Cys220 and Cys248 thiols. βII-Tryptase (1 μm) was incubated with thioredoxin (1 μm), and the reduced enzyme was detected by labeling with MPB at discrete times as described above. The positions of molecular mass standards in kDa are shown at the left. F, plot of the fraction of reduced βII-tryptase as a function of time from experiments of the type shown in E. The inset is the best nonlinear least squares fit of the oxidation data to a single exponential decay. The calculated kobs for the reaction is 0.006 ± 0.002 min−1. The data points and errors are the means ± S.E. of three independent experiments.