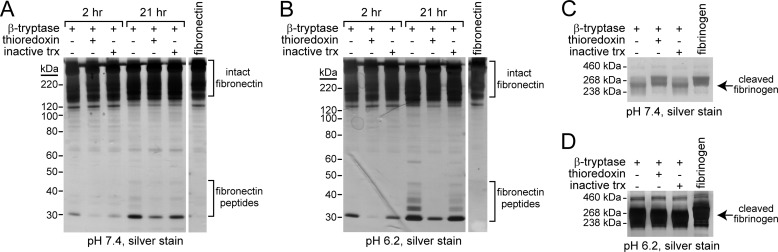
FIGURE 6.
Oxidized and reduced βII-tryptase cleave macromolecular substrates with different efficiency and specificity. A and B, oxidized or reduced β-tryptase was incubated with fibronectin, and the cleavage products were resolved on SDS-PAGE and silver-stained. As a control for the possible effects of thioredoxin on the substrate, βII-tryptase was also incubated with a redox-inactive form of thioredoxin (inactive trx). Oxidized and reduced βII-tryptase produce different fibronectin peptides at both pH 7.4 (A) and 6.2 (B). Intact fibronectin can be seen near the top of the gel, whereas cleaved portions of fibronectin are ∼30–40 kDa in size. C and D, oxidized or reduced β-tryptase was incubated with fibrinogen, and the cleavage products were resolved on SDS-PAGE and silver-stained. Oxidized but not reduced βII-tryptase cleaves fibrinogen at pH 7.4. There is little to no difference in the fibrinogen cleavage products produced by the oxidized and reduced forms of βII-tryptase at pH 6.2.