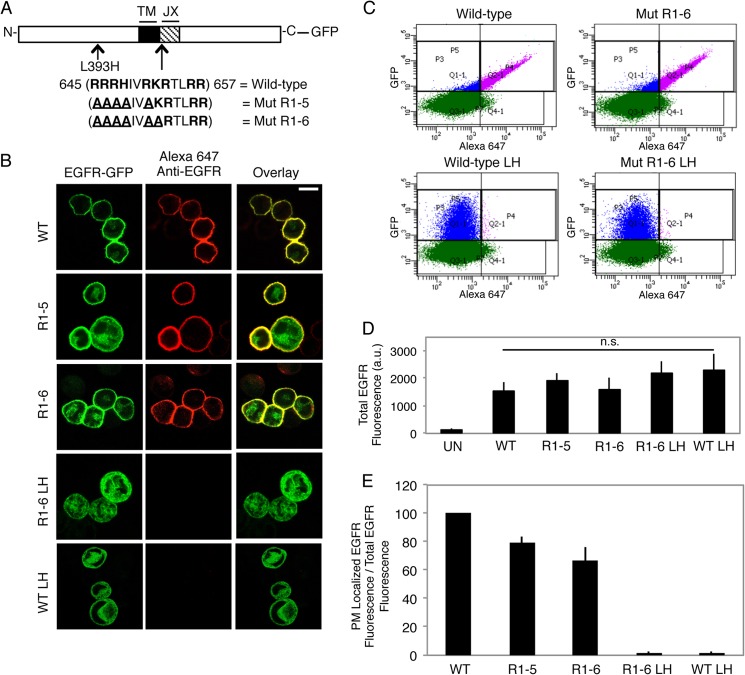
FIGURE 1.
A single point mutation L393H within the N-terminal segment of EGFR inhibits its capacity to traffic to the plasma membrane. A, wild-type and mutant EGFR-GFP constructs generated. The JX sequence in EGFR is shown, with basic amino acids in bold and mutated residues underlined. (TM = transmembrane). B, expression of WT and mutant EGFR-GFP constructs in suspended RBL mast cells. Transiently expressing cells were fixed and labeled with an anti-N-terminal EGFR antibody followed by Alexa 647-anti-IgG. Scale bar, 10 μm. C, representative flow cytometric scatter plots. RBL-2H3 cells transiently expressing GFP-tagged EGFR constructs were harvested, fixed, and labeled with anti-N-terminal EGFR antibody followed by Alexa 647-anti-IgG. Cells were gated on positive GFP fluorescence (blue and purple populations), and Alexa 647 fluorescence was analyzed to determine PM localization (purple population). D, EGFR-GFP constructs express at similar levels. The average GFP fluorescence (total EGFR fluorescence) is from the data plotted in E. UN, untransfected; LH, L393H point mutation; n.s, not significant; a.u., arbitrary units. E, quantification of the effect of mutation of the polybasic JX segment of EGFR and the L393H point mutation (LH) on biosynthetic trafficking. The ratio of PM-localized EGFR fluorescence to total EGFR fluorescence for mutants is normalized to WT, and error bars indicate ± S.D. of three independent experiments in which ≥8000 cells expressing each construct were analyzed.