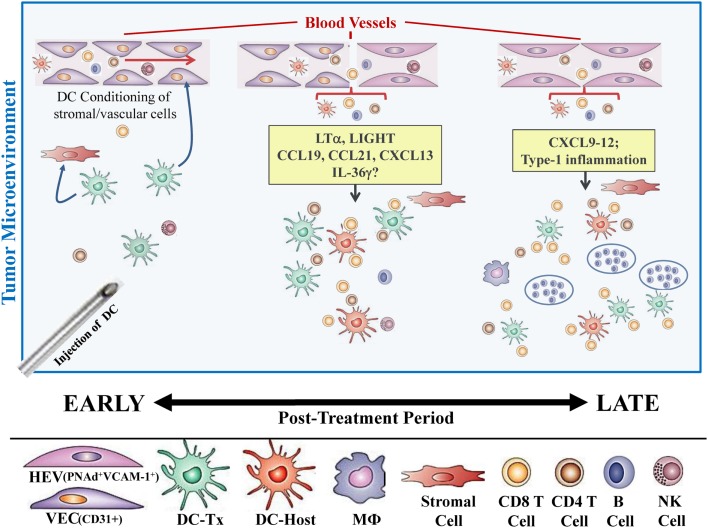
Figure 2.
Hypothetical paradigm for extranodal priming of T cells after intratumoral administration of DC.Tbet cells. Injection of DC.Tbet (but not control DC) into the TME leads to the conditioning of tumor-associated stromal cells and vascular endothelial cells (VEC), resulting in stromal cell production of chemokines recruiting naïve leukocytes (B, T, NK cells) and VEC expression of adhesion molecules, such as VCAM-1, as early as day 2 post-treatment [Figure 1 and (5)]. Recruited lymphocytes are assembled in diffuse patterns around CD11c+ (both injected and host) DC and have already acquired Type-1 functional polarization, based on expression of the Tbet reporter protein (Tbet-ZsGreen) in vivo. PNAd+ HEV are not formally required for early recruitment of naïve T cells into the TME since these structures do not become discernible until later time points [i.e., day 5; Figure 1 and (5)]. B220+ B cells recruited into the TME as a consequence of treatment with DC.Tbet cells are not organized into follicle-like structures during the day 2–5 time period, but may become organized in this manner at even later time points (i.e., ≥day 9 post-therapy), based on previous reports employing alternate immunotherapeutic interventions, such as ch14.18-LTα (8). While therapeutic benefits in our model were largely T cell-dependent and detectable prior to the establishment of formal TLO structures (based on the development of B cell follicles), the presence of “mature” TLO in human tumors has been associated with better clinical prognosis.