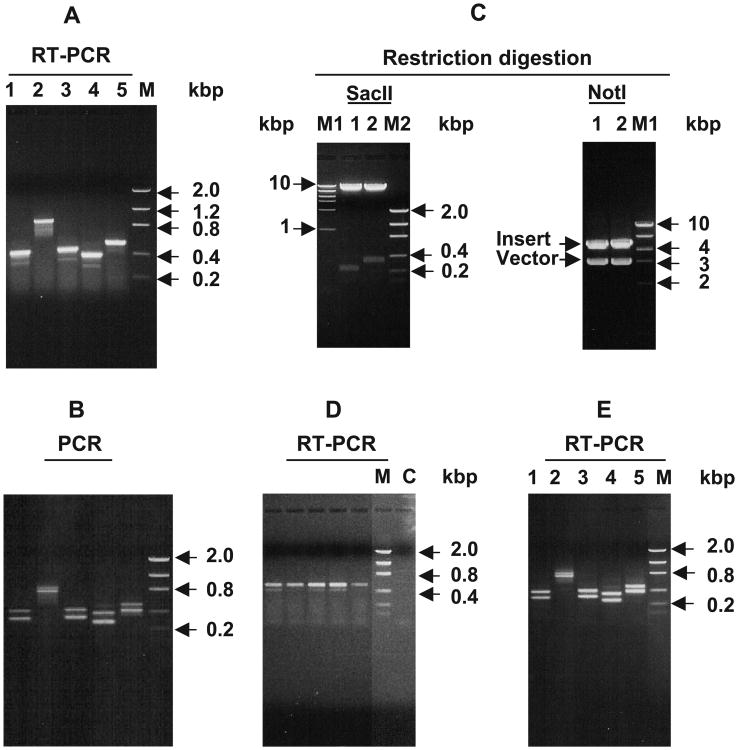
Fig. 3.
Identification of the full-length and spliced forms of the LRAT mRNA transcripts in human liver and testis. (A, B) The presence and absence of the polynucleotide corresponding to the first intron of the LRAT gene in the LRAT mRNA transcripts. Ethidium bromide-stained agarose gel electrophoresis of RT-PCR products of human liver poly[A]+ RNA (A) and of PCR products from the DNA template extracted from the gel shown in Fig. 1C (B). Primer pairs used are as follows: lane 1, primers 8 and 3; lane 2, primers 8 and 4; lane 3; primers 8 and 14; lane 4, primers 8 and 13; lane 5, primers 8 and 9 (see Table 1 for position of the primers). M, low-mass DNA marker. (C) Cloning and analysis of the full-length and spliced form of the human liver LRAT mRNA transcripts. The DNA from the gel shown in lane 3 of Fig. 1C was cut, extracted and cloned by TA cloning as described in Materials and methods. Two clones were picked up for digestion with SacII and NotI enzymes. Lane 1, spliced form clone; lane 2, the intron inclusion form clone. M1 and M2 are high- and low-mass DNA markers, respectively. (D) Expression of LRAT mRNA in individual human liver samples. Ethidium bromide-stained agarose gel electrophoresis of RT-PCR products of total RNA from liver samples, classified as normal, of five individuals. Total RNA from individual samples was extracted and treated with DNase to remove any genomic DNA followed by RT-PCR analysis as described in detail in Materials and methods using primers number 8 and 9 (see Table 1 for sequence and position of the primers). M, low-mass DNA marker; C, negative control. (E) Expression of LRAT spliced forms in human testis. Ethidium bromide-stained agarose gel electrophoresis of RT-PCR products of human testis total RNA using the same sets of primers used in A and B.