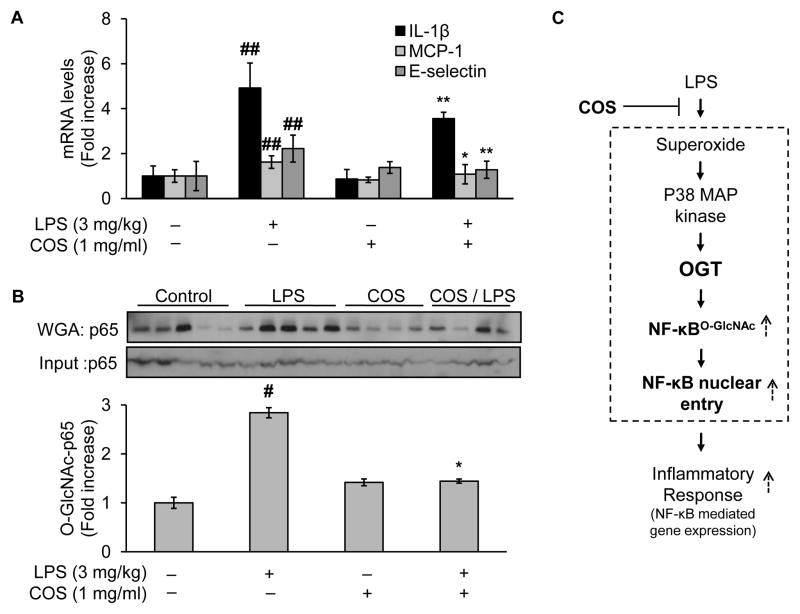
Fig. 8.
Administration of COS inhibits vascular inflammatory response in LPS-treated mice, likely through reduction of LPS-induced NF-κB O-GlcNAcylation. Male C57BL/6J mice (10 weeks of age, 20–30g) were randomly assigned into two major groups: the COS and the non-COS treatment groups (COS-group: 1mg/ml in drinking water, 2 weeks; non-COS group: normal drinking water). Each major group was further divided into two subgroups treated either with LPS or vehicle (LPS from E.coli: 3 mg/kg, i.p.; vehicle: PBS). All mice were euthanized 24h after the acute injection of either LPS/vehicle for tissues collection. Aortic tissues were then subjected to evaluation of (A) mRNA levels of inflammatory cytokines including IL-1β, MCP-1, and E-selectin; and (B) Western blotting of proteins either from WGA-enriched O-GlcNAc modified proteins or whole cell lysates (to assess protein input as a loading control). (C) Proposed mechanism by which COS exerts its anti-inflammatory effects induced by LPS: COS counteracts LPS-elicited vascular endothelial inflammatory response through a mechanism involving OGT-mediated O-GlcNAcylation, nucleus translocation, and activation of NF-κB/p65, both in cultured endothelial cells and experimental mouse model. Text in bold highlighted the new findings in the present study. Data are expressed as means ± SEM. #P<0.05, ##P<0.01 compared to the vehicle-treated control group; *P<0.05, **P<0.01 compared to the LPS-only group.