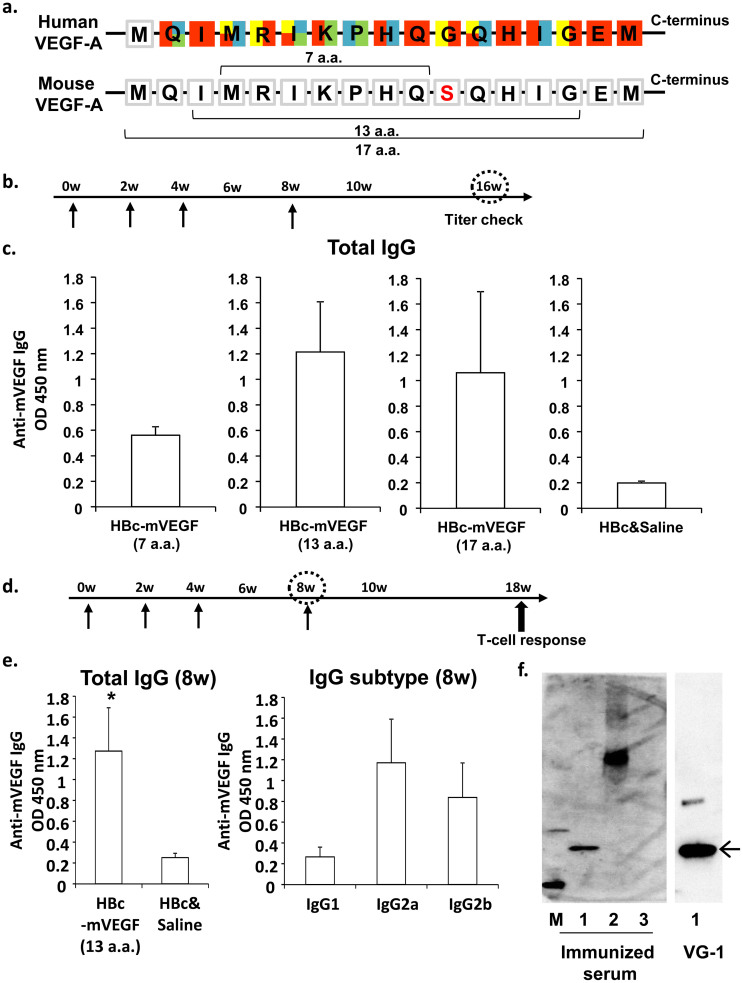
Figure 1. DNA vaccination for VEGF in BALB/c.
(a) Framework residues at VEGF-bevacizumab interface. Red, residues in binding surface with bevacizumab; Yellow, especially important residues of Red; Blue, residues in binding surface with VEGFR-1; Green, residues in binding surface with VEGFR-2. Each residue is represented by single-letter codes. (b) and (d) Time course of DNA vaccination. Vaccination was initially performed using 6 week-old mice (0w), and subsequent vaccinations were given at 2 (2w), 4 or 16 weeks after first vaccination. (c) Titers of anti-VEGF antibodies at 16 weeks. Total IgG titers for VEGF were quantified in mouse sera (100 dilution) from mice immunized with HBc-mVEGF (7 a.a.), HBc-mVEGF (13 a.a.), HBc-mVEGF (17 a.a.) or HBc, respectively. (e) Titers of anti-VEGF antibodies at 8 weeks. Total IgG titers for VEGF were increased only in mouse sera (100 dilution) from the HBc-mVEGF (13 a.a.) group (left panel). The IgG subtype distribution (IgG1, IgG2a or IgG2b) was also evaluated using subtype-specific IgG antibodies in mouse sera (100 dilution) from the HBc-VEGF (13 a.a.) group (right panel). (f) Specific binding of immunized serum to VEGF. Immunized serum used as primary antibody in western blot bound to not only BSA-conjugated mVEGF (13 a.a.) but recombinant mouse VEGF (rmVEGF). Loading samples were as follows; lane 1, recombinant mouse VEGF-A; lane 2, BSA-conjugated mVEGF (13 a.a.); lane 3, BSA-conjugated human Angiopoietin-2 peptide as negative protein. VG-1, commercial monoclonal antibody against VEGF, was used as positive antibody. Data were means ± S.E.M. *p < 0.05 versus control (HBc and Saline).