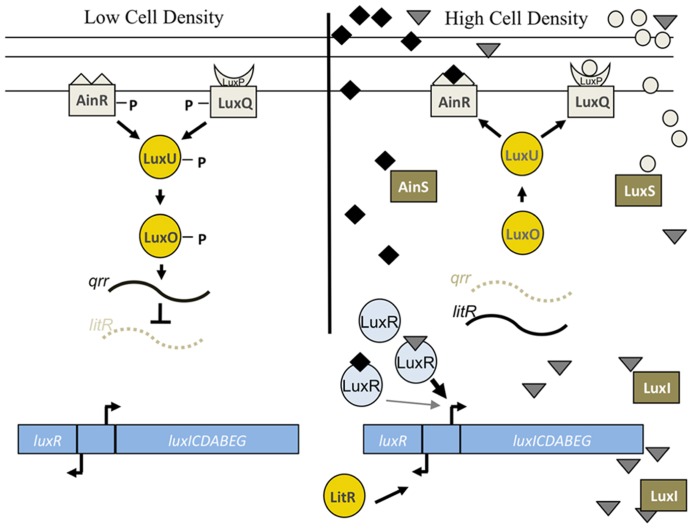
FIGURE 6.
Lux pathway controlling bioluminescence in V. fischeri. At low cell density, the sensor kinases AinR and LuxP/Q are predicted to exhibit net kinase activity leading to the phosphorylation of LuxU and subsequent phosphotransfer to LuxO. Phospho-LuxO induces the expression of the inhibitory sRNA, qrr1, which leads to the degradation of litR mRNA. LitR is the transcriptional activator of luxR, which encodes a protein required for expression of the luxCDEBAG operon. Thus, at low cell density, litR translation is inhibited and the cells do not produce high levels of light. At high cell density, two distinct autoinducer molecules made by AinS (C8-HSL, diamonds) and LuxS (AI-2, circles) are predicted to be at sufficient concentrations to switch the activity of the SKs from net kinase to net phosphatase activity. This leads to dephosphorylation of the downstream regulators, litR translation, and transcription of luxR. LuxR, in conjunction with the autoinducer produced by LuxI (3-oxo-C6-HSL, triangles), leads to the transcription of the lux operon and bioluminescence (reviewed in Stabb et al., 2008). LuxR is also predicted to weakly bind to C8-HSL, which allows for the initiation of luxCDABEG expression. See text for caveats to this model.