Abstract
Background:
This study aims to evaluate the sex hormone binding globulin (SHBG) level as a predictor of response to pharmacological treatment in women with polycystic ovary syndrome (PCOS).
Methods:
This study was conducted in 2009-2012 in Isfahan, Iran. Anovulatory women with a diagnosis of PCOSwere studied. Metformin was started at 500 mg three times a day. If no ovulation occurred, Clomiphene citrate was added.
Results:
The study comprised273 infertile women with PCOS completed the study, 75 (28%) of them became pregnant 6 months after treatment (7.36% with metformin and 20.14% with metformin and clomiphene citrate). Patients who responded to metformin treatment had significantly lower mean SHBG levels compared to those who did not (0.88+0.32vs. 0.2642+0.44 nmol/L, respectively, P<0.0001). The area under the ROC curve (AUC) for prediction the response to treatment was 0.85. The baseline level of 27was the most appropriate cut of point HSBG for the prediction of conception. HSBG had a sensitivity of 88%, and specificity of 73.6%. It had a false positive level of 26.4% and false negative level of 12%. Its positive predictive value was 56.4% and its negative predictive value was 94%. The chance of conception increased for reducing a unit of fpg (OR = 0.69; 95% CI = 0.54-0.86; P = .002), as well as reducing of every unit of HSBG (OR = 0.47; 95% CI = 0.39-0.56; P <0.001), and for reducing each unit of insulin in (OR = 0.082; 95% CI = 1.021-0.33; P <0.001).
Conclusion:
HSBG test is suggested as an appropriate test for predicting pregnancy achievement of PCOs women after pharmacological treatment
Keywords: Binding globulin, metformin, polycystic ovary syndrome, sex-hormone
INTRODUCTION
Polycystic ovarian syndrome (PCOS) is the most common endocrinopathy women, with prevalence of 15.2% in Iranian women of reproductive age based on Rotterdam criteria.[1]
Insulin may act directly and/or indirectly through the pituitary to stimulate ovarian androgen production.[2,3]
The most commonly used definition of PCOS include at least two of the three following criteria: Clinical and/or biochemical sign of hyperandrogenism, oligo and/or anovulation and polycystic ovary on ultrasonography excluding other related diseases such as adrenal congenital hyperplasia, Cushing's syndrome and androgen secreting tumors.[4]
Women with PCOS display decreased levels of sex-hormone binding globulin (SHBG). This glycoprotein, produced in the liver, binds with most sex steroids.
The synthesis of SHBG suppressed by insulin as well as androgens, corticoids, progestins and growth hormone.[5]
Because of supposed SHBG production less circulating androgen is bound and thus more remains available to bind with androgen receptors.
Insulin-sensitizing agents show promise in the treatment of PCOS, when administered to insulin resistant (IR) patients. These compounds act to increase target tissue responsiveness to insulin, thereby reducing the body's need for compensatory hyper insulinemia.
Metformin administered to women with PCOS, increased the frequency of spontaneous ovulation, menstrual cycles and ovulatory response to clomiphene citrate (CC).[6,7]
There is no practical way to predict reliably those who will respond to treatment or not. Preliminary evidence suggests that a response to metformin maybe less likely in women having polymorphism of a gene encoding a hepatic serine-threonine kinase 11 (STK 11).[8]
The purpose of this study is to evaluate the accuracy of measurement of SHBG in women with PCOS as a predictor of response to pharmacologic treatment.
METHODS
The study was conducted from January 2009 to July 2012 in Isfahan, Iran. It was approved by the Ethics committee of Isfahan University of Medical Sciences. Written informed consent was obtained from all participants. They consisted of 180 infertile women diagnosed with PCOS, who were referred to the infertility clinic in a teaching hospital (Shahid Beheshti), affiliated to Isfahan University of Medical Sciences, Isfahan, Iran. The eligibility criteria consisted of normal study of hysterosalpingography, normal semen analysis and age between 18 and 42 years. They were diagnosed retrospectively according to the criteria of the European Society for Human Reproduction and Embryology and the American Society for Reproductive Medicine, i.e., presence of at least two of the following conditions: Chronic anovulation, hyperandrogenism and polycystic ovaries. Menstrual irregularity was assessed as the presence of chronic amenorrhea or the usual cycle length of less than 21 days or more than 35 days or more than 4 days variation between cycles. Hirsutism was defined as the presence of excessive body hair, i.e., Ferriman-Gallwey score >8. Biochemical hyperandrogenism was present if the calculated free androgen index = (Testosterone/[SHBG] × 100) was more than 4.5.
Polycystic ovaries were identified by ultrasonogrophy (ALOKA 1000, 7.5 Hz probe) by either 12 or more follicles with 2-9 mm in diameter or increased ovarian volume >10 ml in at least one of ovaries.
The disease that were included as differential diagnosis of PCOS, as hyperprolactinemia, thyroid dysfunction, late-onset congenital adrenal hyperplasia, androgen secreting tumors and Cushing syndrome were ruled out before starting protocols.
All women underwent anthropometric measurements, including body weight, waist and hip circumferences under standard protocols and by using calibrated instruments. All women underwent a 75 mg oral glucose tolerance test after 8-10 h of overnight fasting. Screening tests were performed on the 2nd days of spontaneous period or progesterone-induced withdrawal bleeding for hormonal profile serum follicle-stimulating hormone-luteinizing hormone Testosterone- and rostenediousedehydroepiandrostrone-sulfate, SHBG, fasting insulin. IR was estimated using the homeostatic model assessment-Insulin resistance (HOMA-IR) (fasting insulin × fasting glucose) × 22.5.
The PCOS women initially received metformin (Chemidarou industrial Co., Tehran, Iran) at 500 mg with a meal one daily in the 1st week and 500 mg twice a day in the 2nd week and 500 mg 3 times a day in the 3rd week and then took this dosage continuously. We used this step of regimen to limit side-effects. We tested liver and kidney before women started taking the study medications. Participants took metformin for at least 3 months unless they become pregnant, if the patient resumed regular menses Transvaginal Sonography (TVS) was performed on the 12 days menstrual cycles for monitoring follicle development, if a dominant follicle (DF) was seen, TVS was repeated every 2 days until the follicle was developed up to 18 mm in diameter.
If patient did not resume regular menses after 3 months of metformin treatment or those with regular menses without DF, 50 mg daily of CC for 5 days was added. On the 5th day of the menstrual cycle, TVS was performed on day 10 from the first day of CC. Human chronic gonadotropin (HCG) was injected the follicle developed up to at least 16 mm in diameter. Intercourse was advised 36 h after HCG injection. CC was increased by 50 mg, 1 day in the next cycle in those cases without response. The maximum dose of CC was 150 mg 1 day for 5 days.
In each women who had no menstruation 3 weeks after timed sexual intercourse, plasma HCG test for pregnancy was performed. Pregnancy was confirmed by plasma HCG level of more than 10 IU/ml and by detection of a gestational sac in the uterus by TVS.
Statistical analysis
Quantitative variable were expressed as mean ± standard deviation (SD) and were compared between groups using one-way analysis of variance (ANOVA) and post-hoc statistical tests. Qualitative variables were expressed as frequency in percent and were analyzed by Chi-square test.
Binary logistic regression was used for quantifying the effect of the predictor variable (waist circumference, hip circumference, body mass index [BMI], SHBG, HOMA-IR, and testosterone). All analyses were performed by SPSS version 20.0 (SPSS, Inc., Chicago, USA). P < 0.05 was considered as statistically significant.
RESULTS
Five of the 273 women with PCOS who were entered into the study were excluded because they suffered from severe gastrointestinal adverse side-effect and had to discontinue the medicine during the 1st week. Of the remaining 268 patients, 75 (28%) became pregnant within 6 months of therapy: 21 women (7.86%) with metformin only and 20 (14%) with metformin and CC. Both responder and non-responder groups for fertility were comparable in terms of age, duration of infertility, proportion of primary/secondary infertility, total ovarian volume, baseline BMI, baseline hormonal and metabolic profile [Table 1]. The mean age of the studied patients was 29.9 ± 6 years with the range of 18-41 years. The mean of BMI index in these patients was respectively 31.3 ± 4.4 and 25.3 ± 4.2, before and after treatment, the mean index decreased significantly (P < 0.001). Table 2 presents the mean value of anthropometric measures before and after the study. It shows that the mean values of waist circumference decreased significantly after treatment (P < 0.001).
Table 1.
Characteristics* of patients who responded or did not responded to treatment
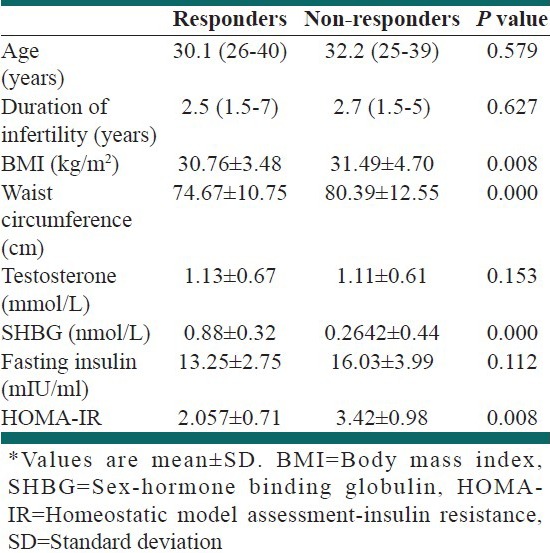
Table 2.
The distribution of anthropometric variables in patients who responded or did not responded to treatment
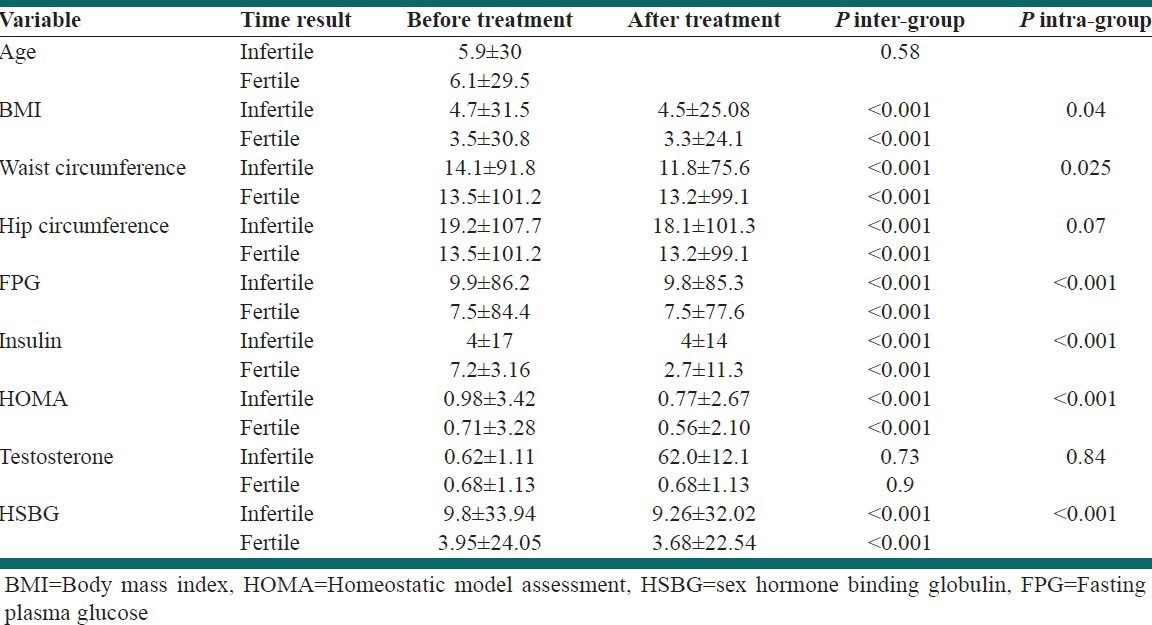
The changes in anthropometric indexes and laboratory tests before and after treatment both groups that responded or did not responded to treatment are shown in Figure 1. The mean (SD) of fasting plasma glucose (FPG), insulin and HOMA-IR changed significantly after treatment. Repeated measure ANOVA test showed that the mean of changes in the mentioned indexes was significantly different between both groups during the treatment period.
Figure 1.
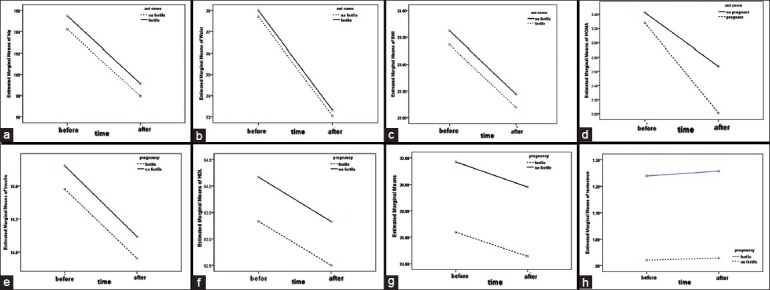
(a) Hip circumferences changes before and after treatment. (b) Waist circumferences changes before and after treatment. (c) Body mass index changes before and after treatment. (d) Homeostatic model assessment changes before and after treatment. (e) Insulin changes before and after treatment. (f) FBG changes before and after treatment. (g) HSBG changes before and after treatment. (h) Testosterone changes before and after treatment
In both groups, the mean (SD) of testosterone level had no significant difference; however, the serum level of sex hormone binding globulin increased significantly after treatment with a significant difference between the two groups studied.
Figure 2 shows the area under the receiver operating characteristic curve area under the curve (AUC) for prediction the response to treatment. Based on the present test, the AUC was 0.85 and confirms that HSBG test is an appropriate test for prediction pregnancy achievement of PCOs women after pharmacologic treatment.
Figure 2.
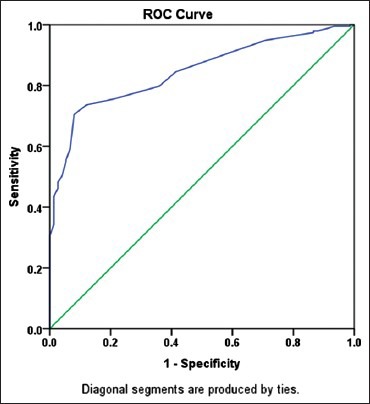
Under receiver operating characteristic curve area for pregnancy prediction by HSBG
The baseline level of 27 was the most appropriate cut point of HSBG for the prediction of conception in these patients. HSBG had a sensitivity of 88% and specificity of 73.6%. It had a false positive level of 26.4% and false negative level of 12%. The positive predictive value of HSBG test (the pregnancy probability of the person with HSBG test level of higher than 27) was 56.4% and its negative predictive value (no pregnancy probability of the person in whom the level of HSBG test was less than 27) was 94% [Figure 3].
Figure 3.
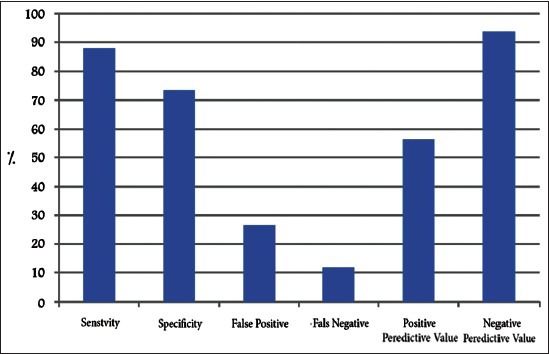
Criteria of the sex-hormone binding globulin marker value for prediction of conception on pharmacological therapy in polycystic ovarian syndrome women
Logistic regression test showed that variables of FPG, SHBG and insulin had significant effects on the pregnancy achievement. Based on these results, the chance of conception increases for reducing a unit of FPG in the rate of 0.68%, which is statistically significant (odds ratio [OR] =0.69; 95% confidence interval [CI] =0.54-0.86; P = 0.002). It also rises for reducing every unit of HSBG in the rate of 0.47% the chances of becoming pregnant for 47.0% (OR = 0.47; 95% CI = 0.39-0.56; P < 0.001). The chance of conception increases for reducing each unit of insulin in the rate of 0.08% (OR = 0.082; 95% CI = 1.021-0.33; P < 0.001) and according to this test, other studied variables had no significant role in prediction of pregnancy conception after pharmacologic therapy and were excluded.
DISCUSSION
To the best of our knowledge, this study is the first report of measuring serum SHBG levels as a predictor of infertile women response with PCOS on pharmacological treatment. We documented that serum SHBG level can be used as an appropriate predictor for response to pharmacological treatment of infertile women with PCOS. It is well-established that IR is a feature of PCOS.[9] IR and the resulting: Raised plasma levels of insulin, are responsible for high androgen concentration in PCOS. Insulin inhibits liver secretion of SHBG and increases the availability of androgens.[10]
Metformin therapy has been shown to have beneficial short-term effects on IR in non-diabetic women with PCOS[11] and its use in women with PCOS in now regarded as acceptable practice.
Our study showed that mean of SHBG levels was 75 ± 0.88 nmol/L in responder women in comparison with 194 ± 0.26 nmol/L in non-responder infertile women. Treatment with metformin can increase the ovulation rates in some women with PCOS.[12] Metformin has been widely used in this regard, but there is no practical way to predict reliably those who will respond. Preliminary evidence suggests that a response to metformin may be less likely in women having a polymorphism of a gene encoding a hepatic STK 11.[8]
Fasting insulin concentrations and glucose: Insulin ratio does not predict response to metformin. It seems that SHBG test can be a predictor to identify the group of women who are more likely to respond to metformin treatment.
It is noteworthy to mention that SHBG is reliable in the non-fasting state and exhibits no diurnal variation, which renders SHBG a unique marker of IR that is useful especially in clinical situations when fasting blood samples are not collected routinely.
Further studies with a larger sample size will be required to validate our findings and to estimate the sensitivity and specificity of this biomarker for identification of responding to metformin as a screening test.
CONCLUSIONS
We found a significant association between SHBG levels and infertility response to pharmacological treatment in PCOS women. This study suggests that assessment of SHBG levels before infertility treatment is a useful tool to identify the group of women who are more likely to respond to treatment.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Mehrabian F, Khani B, Kelishadi R, Ghanbari E. The prevalence of polycystic ovary syndrome in Iranian women based on different diagnostic criteria. Endokrynol Pol. 2011;62:238–42. [PubMed] [Google Scholar]
- 2.Cara JF, Rosenfield RL. Insulin-like growth factor I and insulin potentiate luteinizing hormone-induced androgen synthesis by rat ovarian thecal-interstitial cells. Endocrinology. 1988;123:733–9. doi: 10.1210/endo-123-2-733. [DOI] [PubMed] [Google Scholar]
- 3.Nestler JE, Jakubowicz DJ. Decreases in ovarian cytochrome P450c17 alpha activity and serum free testosterone after reduction of insulin secretion in polycystic ovary syndrome. N Engl J Med. 1996;335:617–23. doi: 10.1056/NEJM199608293350902. [DOI] [PubMed] [Google Scholar]
- 4.Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Bergh C, Carlsson B, Olsson JH, Selleskog U, Hillensjö T. Regulation of androgen production in cultured human thecal cells by insulin-like growth factor I and insulin. Fertil Steril. 1993;59:323–31. doi: 10.1016/s0015-0282(16)55675-1. [DOI] [PubMed] [Google Scholar]
- 6.Nestler JE, Jakubowicz DJ, Evans WS, Pasquali R. Effects of metformin on spontaneous and clomiphene-induced ovulation in the polycystic ovary syndrome. N Engl J Med. 1998;338:1876–80. doi: 10.1056/NEJM199806253382603. [DOI] [PubMed] [Google Scholar]
- 7.Palomba S, Orio F, Jr, Falbo A, Manguso F, Russo T, Cascella T, et al. Prospective parallel randomized, double-blind, double-dummy controlled clinical trial comparing clomiphene citrate and metformin as the first-line treatment for ovulation induction in nonobese anovulatory women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90:4068–74. doi: 10.1210/jc.2005-0110. [DOI] [PubMed] [Google Scholar]
- 8.Fritz MA, Speroff L. Clinical Gynecologic Endocrinology and Infertility. 8th ed. London: Lippincott Williams and Wilkins; 2011. Chronic anovulation and the polycystic ovary syndrome; p. 525. [Google Scholar]
- 9.Dunaif A. Insulin resistance and the polycystic ovary syndrome: Mechanism and implications for pathogenesis. Endocr Rev. 1997;18:774–800. doi: 10.1210/edrv.18.6.0318. [DOI] [PubMed] [Google Scholar]
- 10.Burghen GA, Givens JR, Kitabchi AE. Correlation of hyperandrogenism with hyperinsulinism in polycystic ovarian disease. J Clin Endocrinol Metab. 1980;50:113–6. doi: 10.1210/jcem-50-1-113. [DOI] [PubMed] [Google Scholar]
- 11.Moghetti P, Castello R, Negri C, Tosi F, Perrone F, Caputo M, et al. Metformin effects on clinical features, endocrine and metabolic profiles, and insulin sensitivity in polycystic ovary syndrome: A randomized, double-blind, placebo-controlled 6-month trial, followed by open, long-term clinical evaluation. J Clin Endocrinol Metab. 2000;85:139–46. doi: 10.1210/jcem.85.1.6293. [DOI] [PubMed] [Google Scholar]
- 12.Kocak M, Caliskan E, Simsir C, Haberal A. Metformin therapy improves ovulatory rates, cervical scores, and pregnancy rates in clomiphene citrate-resistant women with polycystic ovary syndrome. Fertil Steril. 2002;77:101–6. doi: 10.1016/s0015-0282(01)02941-7. [DOI] [PubMed] [Google Scholar]


