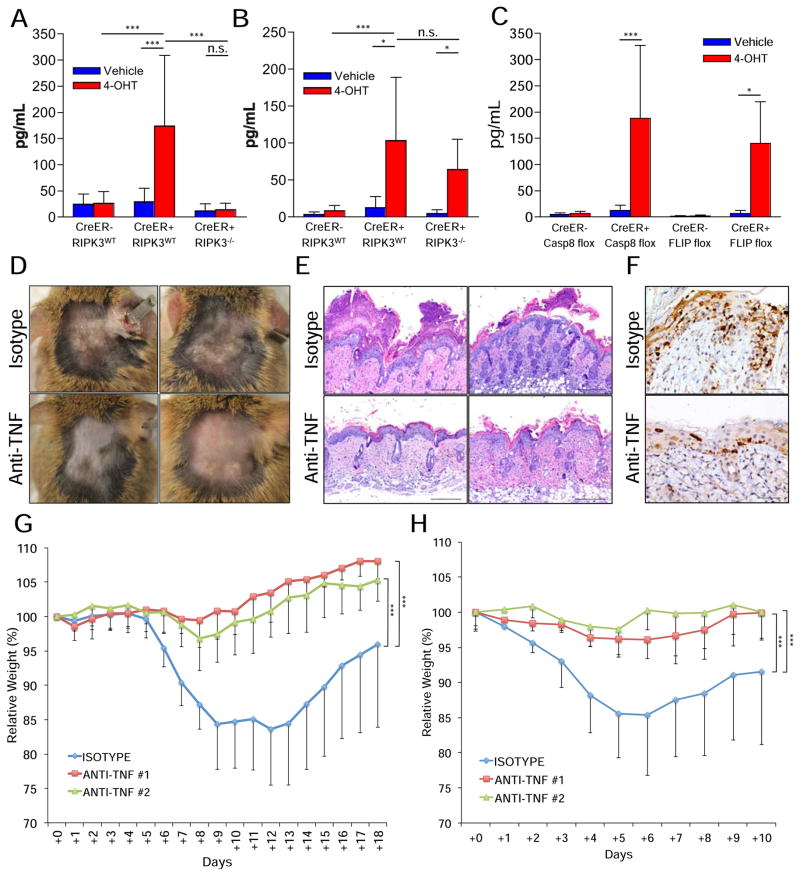
Figure 4. Neutralization of TNF protects from the deleterious effects of caspase-8 or FLIP acute deletion.
(A–E) Animals were shaved in two distinct dorsal areas. The shaved area around the neck was painted with 4-hydroxytamoxifen (4OHT) while the shaved area near the tailbase was painted only with vehicle. (A–B) IL-1b levels in the skins of (A) Casp8f/f animals at day +10 or (B) cflarf/f animals at day +5. (C) TNF levels in the skins of casp8f/f (Casp8 flox) animals at day +10 or cflarf/f (FLIP flox) animals at day +5. (D–F) CreER+, cflarf/f animals were i.p. injected at days −1, +1 and +3 with 0.5mg of anti-TNF antibodies (clone XT3.11) or isotype control antibodies (clone HRPN) and examples of (D) photos, (E) sections stained with hematoxylin and eosin (40X) and (F) cleaved caspase-3 staining sections (10X) were produced at day +8. (G) CreER+, casp8f/f animals were gavaged with 1mg tamoxifen per 25g animal body weight for 6 consecutive days and i.p. injected at days +0, +2 and +4 with 0.5mg of two different neutralizing anti-TNF antibodies (#1 = XT3.11, #2 = HB10649) or isotype control (clone HRPN). Weight loss was followed for 18 days. (H) CreER+, cflarf/f animals were treated as in (G), but gavaged only twice, at days +0 and +2, and their weights were tracked for 10 days. (*) p<0.05. (***) p<0.001. (n.s.) not statistically significant.